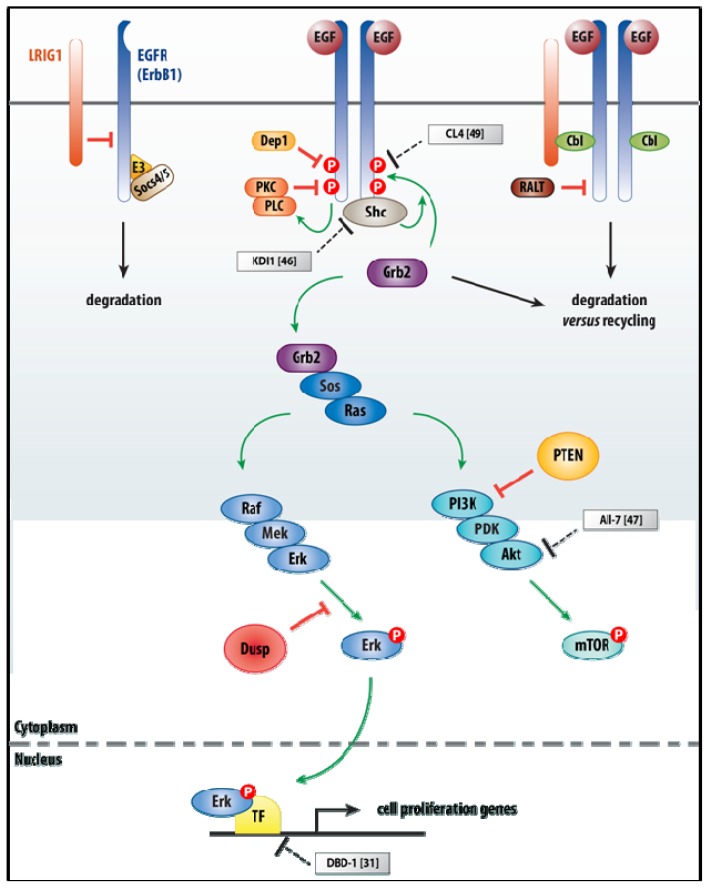
Figure 1.
An overview of signaling by the EGF family (with EGF and EGFR/ErbB1 as example) from cell surface down to the nucleus (without taking into account endocytic trafficking and recycling of the receptor). In the absence of ligand (left panel), LRIG1, a negative regulator (red line), binds to the EGFR and both are degraded by less well-studied mechanisms. In addition, unliganded EGFR can also be sent into E3 ubiquitin ligase-Socs4/5-assisted ubiquitylation and degradation. In the presence of ligand (middle and right panel), still two outcomes are possible. In the first, positive signaling (green arrows) is initiated via Grb2, resulting in activation of downstream kinase cascades Raf–Mek–Erk (Erk or Mapk) and PI3K–PDK–Akt, which is a prototypic survival pathway that is constitutively activated in many types of cancer. The latter cascades are also subject to negative regulation exerted by dual-specificity phosphatases (Dusp) and the tumor-suppressor PTEN, respectively. In the nucleus, Erk teams up with an activated TF to induce transcription of cell proliferation genes. In the second, degradation versus recycling is still an option, one initiated via Grb2 and one—also in a ligand-dependent manner—where LRIG1-Cbl (Cbl is an E3 ubiquitin–protein ligase) and probably direct EGFR–Cbl interaction ensure full ubiquitylation and hence degradation of the EGFRs. Finally, also RALT, a pan-ErbB inhibitor, acts at this level: it inhibits EGFR allosteric kinase activation. Grey boxes highlight the aptamers discussed in the main text, together with the references.