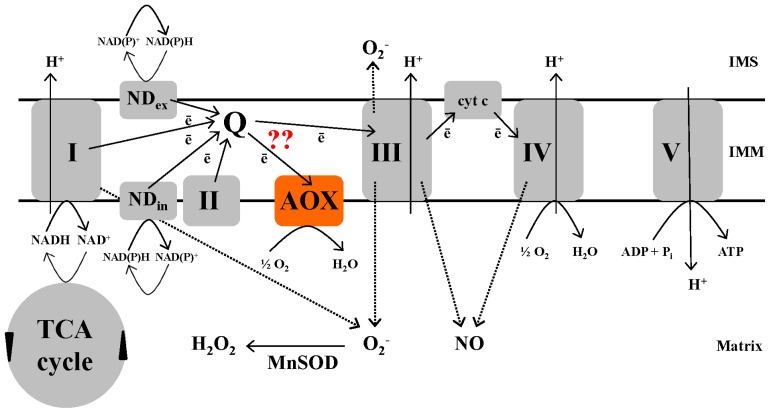
Figure 1.
The plant mitochondrial electron transport chain. NADH oxidation by complex I is coupled to proton transport from the matrix to IMS, while NAD(P)H oxidation by a series of alternate dehydrogenases is not coupled to proton transport. Similarly, electron flow from ubiquinol to complex IV (reducing O2 to H2O) is coupled to proton transport (at two sites) while electron flow from ubiquinol to AOX (also reducing O2 to H2O) is not coupled to proton transport. Proton transport generates a proton motive force that is subsequently dissipated by ATP synthase (complex V) to produce ATP. Plants can therefore modulate their ATP yield depending on the components of the ETC being used for NAD(P)H oxidation and O2 reduction. When the ability of an ETC component to transport electrons is reduced and/or membrane potential is high, electron transport can slow, leading to an over-reduction of the ETC. Under these conditions, single electron leak to O2 or nitrite increases, producing O2− and NO, respectively. In plants, the specific sites and mechanisms of O2− and NO generation are not yet well understood. See text for further details. I, II, III, IV, V: complexes I to V, IMS, inner membrane space; IMM, inner mitochondrial membrane; MnSOD, manganese superoxide dismutase; NDin, internal-oriented alternate NAD(P)H dehydrogenases; NDex, external-oriented alternate NAD(P)H dehydrogenases; Q, ubiquinone pool.