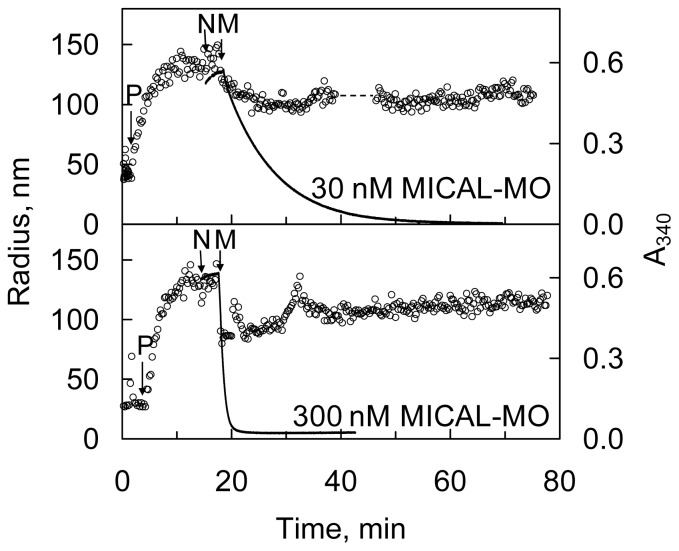
Figure 15.
Monitoring the changes of the aggregation state of actin in solution in the presence of human MICAL-MO and NADPH by dynamic light scattering. Polymerization of G-actin (5 μM) was induced by adding 1/10 volume of polymerization buffer (P) at 25 °C. The mean radius of the particles in solution was determined with a Dynapro MS/X instrument (Protein Solutions, Lakewook, NJ, USA) by acquiring signals every 15 s (○). NADPH (100 μM, N) and MICAL-MO (M, 30 or 300 nM) were added at the indicated times and the reaction was monitored by dynamic light scattering (DLS). Solid lines: oxidation of NADPH (100 μM) monitored at 340 nm under identical conditions. The A340 traces are offset to match the time of enzyme addition to the solution containing F-actin and NADPH in the DLS experiment. Reprinted from [70] with permission from Elsevier.