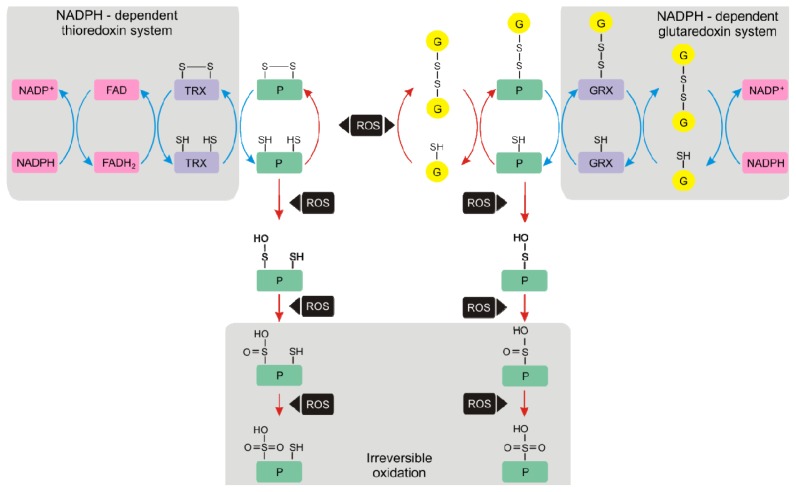
Figure 2.
Thiol-disulphide cycle, proposed to contribute to the biochemical mechanisms that confer desiccation tolerance, involving glutathionylation of proteins, GRX and TRX. This figure is reproduced from Colville and Kranner [14], with the permission of Plant Growth Regulation. Desiccation tolerant organisms can lose more than 90% of their water without dying and revive upon rehydration, and extreme fluctuations in water content are accompanied by equally extreme changes in cellular redox state, associated with an increase in ROS levels. Red arrows: processes that may occur predominantly during desiccation; blue arrows: processes that occur primarily during rehydration. The thiol (-SH) groups of redox-active Cys residues in proteins (P; green) and glutathione (G; yellow) are susceptible to oxidation, and in the dry state, a shift from thiols to the disulphide forms occurs as the probability for enzymatic reduction decreases with progressive water loss. Glutathionylation protects protein Cys residues from further oxidation to sulphenic (PSOH), sulphinic (PSO2H) and sulphonic (PSO3H) acids. Sulphenic acid can be reduced by glutathione, whereas sulphonic and sulphinic acid formation are thought to be irreversible. Glutathionylation may occur through several mechanisms, for example, through reactions between a protein thiol (PSH) and GSSG, forming protein-bound glutathione (PSSG). This will occur under conditions of severe oxidative stress when GSSG accumulates. Other mechanisms include reactions of protein thiyl radicals or sulphenic acid intermediates with GSH; glutaredoxins (GRX) can also catalyse the reaction between GS• and PSH to produce PSSG (not shown), although GRXs normally act as reductants in de-glutathionylation reactions. Upon rehydration, the reduction of protein disulphides can be catalysed by thioredoxin (TRX), which is subsequently reduced by TRX reductases (NADPH-dependent thioredoxin system). The NADPH-dependent thioredoxin system operates in the cytosol and mitochondria, where TRX reductases utilise electrons supplied by NADPH, which are transferred to TRX disulphide via flavoproteins (FAD). In chloroplasts, TRX reductases may use ferredoxin as an electron donor. Moreover, upon rehydration GRXs can reduce PSSG, leading to the formation of a mixed disulphide between GSSG and GRX. This is reduced by GSH, and the resulting GSSG is reduced by GR (NADPH-dependent glutaredoxin system). The active site of GRXs can either have one (monothiol) or two (dithiol) catalytic Cys residues; only the monothiol mechanism for PSSG reduction is shown.