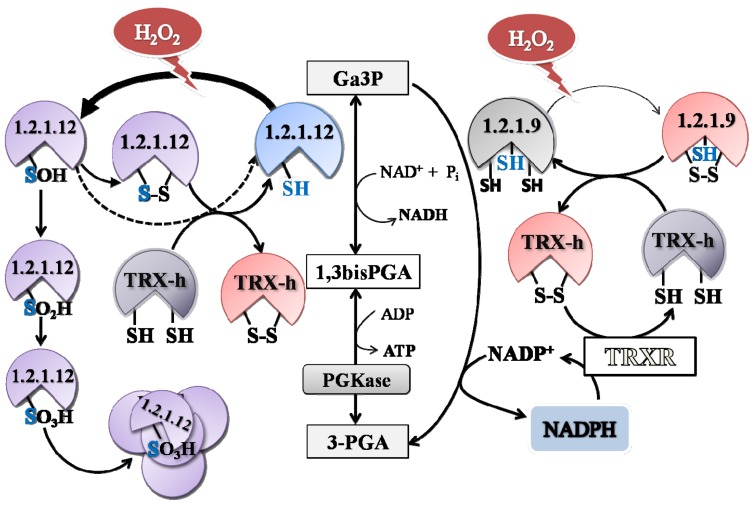
Figure 7.
Scheme proposed for np-Ga3PDHase and Ga3PDHase oxidation. Redox regulation of Ga3PDHase (EC 1.2.1.12) could probably take place by routes involving one or more cystein residues. When two thiol groups are involved, a disulfide bridge would occur; but, as results partially support, some Cys would escape disulfide formation and become irreversibly oxidized by formation of sulfenic (SOH), sulfinic (SO2H) and sulfonic (SO3H) acid, depending on the oxidant involved, its concentration and the extent of the oxidation. Reduction of Ga3PDHase by TRX-h could be possible from the disulfide bridge (eventually also from sulfenic acid, dashed arrows), but the oxidation to sulfinic and sulfonic acids turns irreversible and could promote protein aggregation and precipitation. By the other hand, redox regulation of np-Ga3PDHase (EC 1.1.1.9) would occur by disulfide bond formation probably between two Cys residues different from the catalytic one (marked in blue), which can be reverted anytime by reduced TRX-h. The synthesis of NADPH by np-Ga3PDHase under the incidence of an oxidative situation, would favor the activity of antioxidant systems (like thioredoxin (TRX) which involves the thioredoxin reductase (TRXR) that maintains TRX-h in its reduced state). This would feedback NADPH production by maintaining the np-Ga3PDHase in its reduced (active) state.