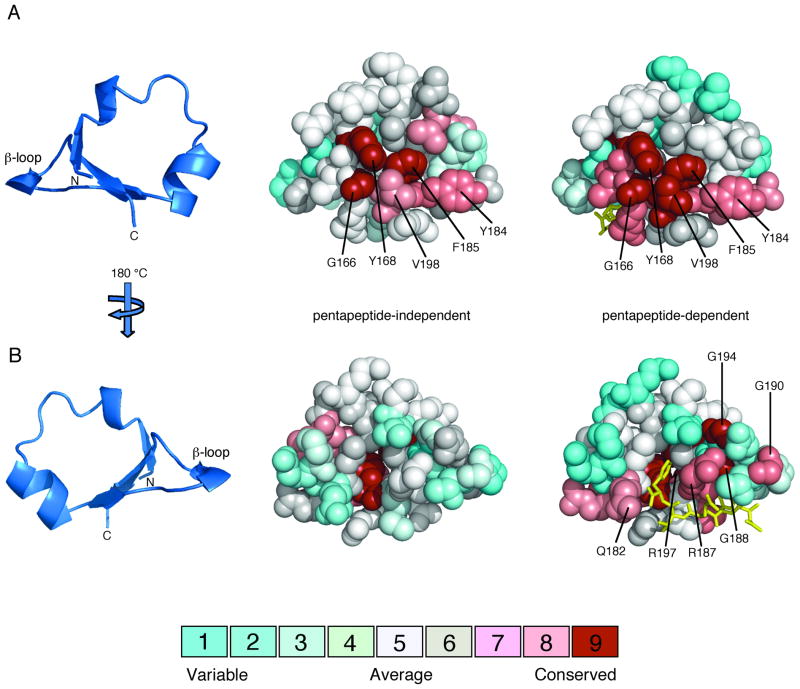
FIG. 4.
Amino acid conservation on the surfaces of pentapeptide-independent and pentapeptide-dependent β-subdomains. Multiple sequence alignments of pentapeptide-independent and pentapeptide-dependent CheR β-subdomains, classified in Figure 3, were generated using ClustalW (Thompson et al., 1994). Amino acid conservation scores for each residue were calculated using the Consurf maximum likelihood method (Glaser et al., 2003) (http://consurf.tau.ac.il) and were mapped onto space-filling models of the CheR β-subdomain (PDB accession code 1BC5) (Djordjevic and Stock, 1998) using Pymol (Delano, 2002). The coloring scheme for conservation ranges from cyan (variable) to red (conserved) and the pentapeptide (gold) is shown in stick representation. A. β-subdomain interior face. The ribbon diagram of the β-subdomain (left) indicates the orientation of the space-filling models, viewed toward the surface that packs against the catalytic domain of CheR. Residues that are highly conserved in both pentapeptide-independent (center) and pentapeptide-dependent (right) β-subdomains are labeled. B. β-subdomain exterior face. The orientation corresponds to a 180° rotation around a vertical axis relative to the view in A. Residues that are highly conserved only in pentapeptide-dependent β-subdomains (right) are labeled.