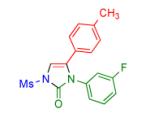
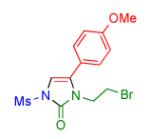
Table 2.
Transannulation of 1-Mesyl-1,2,3-triazoles with Isocyanatesa
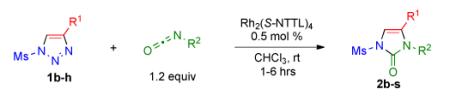
| entry | product | yield, %b |
entry | product | yield, %b |
entry | product | yield, %b |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |

|
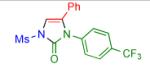
2b | 95 | 7 |
|
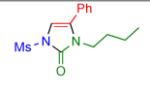
2h | 83 | 13 |
|
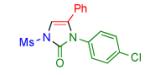
2n | 97 |
| 2 |
|
2c | 94 | 8 |
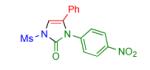
|
2i | 53 | 14 |
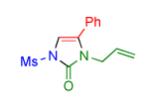
|
2o | 82 |
| 3 |
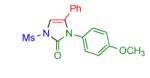
|
2d | 94 | 9 |
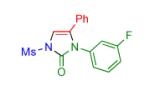
|
2j | 89 | 15 |
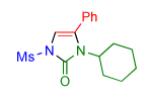
|
2p | 95 |
| 4 |
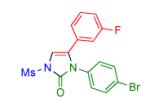
|
2e | 94 | 10 |
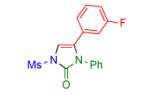
|
2k | 92 | 16 |
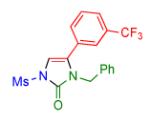
|
2q | 93 |
| 5 |
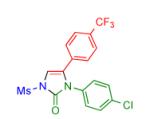
|
2f | 80 | 11 |
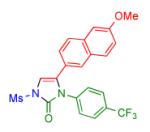
|
2l | 69 | 17 |
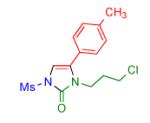
|
2r | 90 |
| 6 |
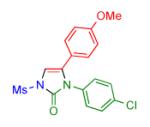
|
2g | 85 | 12 |
|
2m | 85 | 18 |
|
2s | 92 |
Procedure: triazole 1 (1.0 mmol), isocyanate (1.2 mmol), Rh2(S-NTTL)4 (0.005 mmol) were stirred in 5 mL of dry chloroform at room temperature for 1-6 hours.
Isolated yield.
