Abstract
Multiple studies show elevations of α, β-unsaturated aldehydic by-products of lipid peroxidation including 4-hydroxynonenal and acrolein in vulnerable brain regions of subjects throughout the progression of Alzheimer’s disease (AD). More recently 4-hydroxyhexenal (HHE), a diffusible α, β-unsaturated aldehyde resulting from peroxidation of ω-3 polyunsaturated fatty acids, was shown to be elevated in the hippocampus/parahippocampal gyrus (HPG) of subjects with preclinical AD (PCAD) and in late stage AD (LAD). HHE treatment of primary rat cortical neuron cultures led to a time- and concentration-dependent decrease in survival and glucose uptake. To determine if HHE also impairs glutamate uptake, primary rat astrocyte cultures were exposed to HHE for 4 hours and glutamate transport measured. Results show subtoxic (2.5 μM) HHE concentrations significantly (p < 0.05) impair glutamate uptake in primary astrocytes. Immunoprecipitation of excitatory amino acid transporter-2 (EAAT-2), the primary glutamate transporter in brain, from normal control (NC), mild cognitive impairment (MCI), PCAD and LAD HPG followed by quantification of HHE immunolabeling showed a significant increase in HHE positive EAAT-2 in MCI and LAD HPG. Together these data suggest HHE can significantly impair glutamate uptake and may play a role in the pathogenesis of AD.
Keywords: Lipid peroxidation, 4-hydroxyhexenal, glutamate transport, excitatory amino acid transporter-2
Introduction
Increasing evidence supports a role for oxidative damage to lipids, protein and nucleic acids in the pathogenesis of several neurodegenerative disorders including Alzheimer’s disease (AD). Multiple studies show increased DNA (reviewed in[1], RNA [2–4], and protein oxidation (reviewed in [5] and lipid peroxidation [6–9] in vulnerable regions of brain from late stage AD (LAD) subjects compared to normal control (NC) subjects. In addition, markers of oxidative damage are elevated in subjects with mild cognitive impairment (MCI), the earliest clinical manifestation of AD (reviewed [1] as well as in subjects with preclinical AD (PCAD), a condition in which subjects show normal ante-mortem neuropsychological test scores but significant AD pathology at autopsy [10–13] Interestingly, levels of oxidative damage early in disease progression are comparable to those observed in late stage disease suggesting oxidative damage is an early event in the pathogenesis.
The high polyunsaturated fatty acid content of brain coupled with the relative paucity of antioxidants make lipid peroxidation a likely event. In addition to impaired membrane integrity, lipid peroxidation leads to production of a variety of by-products including isoprostanes and neuroprostanes, straight chain aldehydes and α, β-unsaturated aldehydes. Although isoprostanes and neuroprostanes do not show toxicity, they serve as excellent markers of arachidonic and docosahexaenoic acid peroxidation in brain and CSF [14]. F2-isoprostane (F2-IsoP) levels in CSF also may be useful as a monitor of the efficacy of antioxidant treatment in AD (reviewed in [14]). In contrast to isoprostanes and neuroprostanes, α, β-unsaturated aldehydes including acrolein, 4-hydroxynonenal (HNE) and 4-hydroxyhexenal (HHE) are neurotoxic in addition to being markers of lipid peroxidation. The electrophilic nature of these aldehydes make them highly reactive with sulfhydryl groups of cysteine, lysine and histidine [15–18] potentially altering normal protein function. Several previous studies show levels of acrolein and HNE are elevated in late-stage AD brain [8, 19, 20] and CSF [21]. More recently, we used GC/MS with negative chemical ionization to show significant elevations of levels of a third α, β-unsaturated aldehyde, 4-hydroxyhexenal (HHE), in vulnerable regions of PCAD and LAD brain and significant elevations of protein bound HHE in PCAD, MCI and LAD HPG[22]. Multiple in vitro studies demonstrate HNE and acrolein are neurotoxic [23–27] and can significantly impair proteins critical for cell survival including significant impairment of glucose transport [13, 23, 24], Na/K ATPase activity [7] and glutamate transport [23, 24]. Although HHE has only recently been the focus of study in AD brain it may be of importance in the pathogenesis of AD because it is the by-product of peroxidation of ω-3 polyunsaturated fatty acids including docosahexaenoic acid (DHA), the predominant PUFA in grey matter [15, 16]. Concentrations of DHA are 30 – 50 times those of arachadonic acid, the predominate ω-6 PUFA, suggesting that oxidative stress in brain would likely lead to increased generation of HHE.
Previous studies show HHE is toxic to cerebral cortical neurons with an LD50 comparable to that of HNE [28] and that HHE/glutathione adducts are prominent in biomarkers of lipid peroxidation in rats subjected to ethanol withdrawal [29]. In our previous studies we showed HHE led to a time- and concentration-dependent decrease in survival of primary rat cortical neuron cultures and significant impairment of glucose uptake [22]. In the current study we measured the effects of HHE on glutamate transport in primary rat astrocyte cultures. To determine if HHE is associated with proteins critical for glutamate transport in brain we immunoprecipitated excitatory amino acid transporter 2 (EAAT2), the primary glutamate transporter in brain, and quantified levels of HHE modification.
Materials and Methods
Specimens of hippocampus/parahippocampal gyri (HPG) were obtained from short post mortem interval (PMI) autopsies of 8 normal control (NC) subjects, 8 subjects with mild cognitive impairment (MCI), 8 preclinical AD (PCAD) and 7 late-stage AD (LAD) subjects through the neuropathology core of the University of Kentucky Alzheimer’s Disease Center (UK-ADC). Normal control and PCAD subjects were followed longitudinally in the UK-ADC Normal Control Clinic and underwent memory testing, physical and neuropsychological testing yearly. These subjects showed no evidence of memory decline and had antemortem neuropsychological test scores in the normal range when corrected for age and education. Subjects with MCI were normal on enrollment in the UK-ADC longitudinal study but developed MCI during follow-up. Clinical criteria for MCI are as described by Peterson et al. [30]. Late-stage AD subjects met the clinical criteria for probable AD as described by McKhann et al. [31]. Neuropathological examination of multiple sections of neocortex, hippocampus, entorhinal cortex, amylgdala, basal ganglia, nucleus basalis of Meynert, midbrain, pons, medulla and cerebellum using the modified Bielshowsky stain, hematoxylin and eosin stains and amyloid beta peptide (Aβ) and α-synuclein immunostains was carried out for all subjects by the Neuropatholgy Core of the UK-ADC. Braak staging scores were determined using the Gallays stain on sections of entorhinal cortex, hippocampus and amygdala and the Bielshowsky stain on neocortex. Histopathologic examination of NC subjects showed only age-associated changes with a median Braak staging score of I. Subjects with MCI showed a significant increase in neuritic plaques in neocortical regions, and a significant increase of NFT in entorhinal cortex, HPG and amygdala compared to NC subjects [32] with a median Braak score of III. PCAD subjects met National Institute on Aging-Reagan Institute [33] criteria for the histopathologic diagnosis of AD with moderate or frequent neuritic plaques in neorcortex and a median Braak staging score of IV. LAD subjects met accepted guidelines for the histopathologic diagnosis of AD with a median Braak score of VI. None of the subjects analyzed demonstrated significant Lewy body pathology.
Tissue Processing and Immunoprecipitation of HHE positive EAAT2
To determine if EAAT-2 is significantly altered in the progression of AD, specimens of HPG from NC, PCAD, MCI and LAD subjects were homogenized using a cold Dounce homogenizer and RIPA buffer containing 50 mM Tris HCl, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, (pH 8) and complete protease inhibitors (Roche, Madison, WI, USA). The homogenate was then centrifuged at 5,000 × g and protein content of the supernatant determined using the Pierce BCA method per manufacturer’s instructions. Two hundred μg samples of each specimen were subjected to immunoprecipitation using a polyclonal antibody against EAAT2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) using the method of Wiltfang et al., [34] with modification. Briefly, rabbit anti-EAAT-2 antibody was covalently cross linked to Protein A/G Plus Sepharose beads (Santa Cruz Biotechnology, Santa Cruz, CA, USA) by incubation at room temperature for 2 h. The beads were centrifuged at 1,000 × g for 3 min and washed 3 to 5 times with 500 μl PBS (pH 7.2). The beads were then added to the protein aliquots and incubated overnight on a rotation plate at 4 °C. The beads were isolated by centrifugation, rinsed 3 times with PBS and suspended in 30 μl gel loading buffer. Immunoprecipitated proteins were eluted by heating at 100 °C in gel loading buffer, separated using a 10 to 20% gradient gel and subjected to Western blot analysis using anti-HHE antibody (Genox, Baltimore, MD, USA). The immunoprecipitation blank consisted of all reagents without protein. In addition, 20 μg aliquots of tissue homogenate were subjected to SDS/PAGE and levels of EAAT2 determined using the same poly clonal antibody. Analysis of levels of total EAAT2 showed a slight but significant (p < 0.05) decrease in LAD HPG. Therefore results of the immuoprecipitation studies were normalized to levels of total EAAT2 for each sample. Each individual ratio was then normalized to the mean ratios for NC subjects. Results are expressed as mean ± SEM % control values. Our previous study showed the HHE antibody provides a linear response to increasing levels of HHE-modified protein and that the antibody is specific to HHE and does not cross react with HNE or acrolein [13].
Primary Astrocyte Cultures
Primary astrocyte cultures were established from cerebral hemispheres of 2 day old Sprague-Dawley rats as described by Keller et al. [35] using University of Kentucky IACUC approved protocols. Astrocytes were plated in uncoated 60 mm dishes and maintained in MEM supplemented with 26 mM sodium bicarbonate, 10 mM glutamine and 10% (v/v) fetal bovine serum. Astrocyte cultures prepared using this method are >98% type I ascrocytes as assessed by anti-glial fibrillary acid protein staining [35]. For treatment, astrocytes 2 weeks in culture were switched to serum free Locke’s solution consisting of 154 mM NaCl, 5.6 mM KCl, 2.3 mM CaCl2, 3.6 mM NaHCO3, 10 mM glucose, 5 mM HEPES (pH 7.2) with 10 mg/L gentamicin sulfate and treated with vehicle, 0.5 μM, 1.0 μM, 2.5 μM, 5 μM, 10 μM and 25 μM HHE for 4 hours. Viability of cultures following treatment was assessed by measuring lactate dehydrogenase release into the medium as described by Koh and Choi [36] or by measurement of reduction of 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) as a measure of mitochondrial viability as described by Mossman et al. [37].
Glutamate Uptake
Glutamate uptake as assayed as described by Swanson [38] with modification. Primary astrocytes were switched to Locke’s with glucose and treated with increasing concentrations of HHE for 4 hours. After treatment, 3H-labeled glutamate (3,4-[3H] glutamic acid, 44.0 Ci/mmol; 22.7 mM) (NEN life Science Products, Boston, MA, USA) was added at a final concentration of 23 μM for 5 minutes. Previous studies demonstrate linearity of uptake for glutamate for comparable periods of time [39]. Uptake was terminated by rapid aspiration of the medium and rinsing 3 times with cold PBS. Cells were then lysed in 250 μl 0.5M NaOH/0.5% SDS and scraped. Aliquots (20 μl) were taken in duplicate for protein determination using the Pierce BCA method. For quantification of radio-labeled glutamate taken up by cell cultures, 160 μl aliquots of cell lysate were added to 5 ml liquid scintillation cocktail (Scintiverse E; Fisher, St. Louis, MO) and counted using a Packard 2500 liquid scintillation counter. Blanks for the assay consisted of cultures exposed to 200 μM unlabeled (cold) glutamate concomitantly with radio-labeled glutamate. Results are expressed as mean ± SEM % control glutamate and are the mean of 9 to 15 dishes.
Statistical Analyses
Statistical analyses were carried out for cell culture survival and glutamate transport using analysis of variance with Dunnett’s post hoc test for individual differences and the commercially available ABSTAT (AndersonBell, Arvada, CO, USA) software. Levels of HHE immunopositive EAAT-2, and subject age and PMI were compared using ANOVA with Dunnett’s post hoc test. Braak staging scores were compared using the Mann Whitney U-test and are expressed as median scores.
Results
Table 1 shows demographic data for subjects analyzed in the IP experiment and shows no significant differences in age or PMI for any of the subject groups. Braak staging scores were significantly higher in MCI (median = III), PCAD (median = IV) and LAD (median = VI) subjects compared to NC subjects (median = I). Figures 1A and 1B show representative Western blots of total EAAT2 (Figure 1A) and HHE-positive EAAT2 following immunoprecipitation (Figure 1B) from NC, MCI, PCAD and LAD HPG. Figure 1C shows the negative control IP reaction and shows virtually no non-specific reactivity. Results of quantification of immunostaining of Figures 1A and 1B are shown in Figure 2 and show a significant decrease of EAAT2 in disease progression with a significant decrease in LAD HPG compared to NC HPG. Figure 2 also shows a significant disease related increase in levels of HHE positive EAAT2 with significant (p < 0.05) increases in MCI and LAD HPG compared to NC HPG. To ensure the slight decreases in total EAAT2 levels did not alter results of HHE measures, levels of HHE-modified EAAT2 were normalized to total EAAT2 and compared using ANOVA. Figure 2 shows normalization of HHE positive EAAT2 to total EAAT2 leads to similar results to those observed for HHE-positive EAAT2 with significant elevations of HHE-positive EAAT2 in MCI and LAD HPG compared to NC HPG. Consistent with previous studies, levels of HHE positive EAAT-2 were similarly elevated for MCI and LAD subjects suggesting HHE modification of the protein occurs early in disease progression.
Table 1.
Subject Demographic Data
| Mean ± SEM Age (y) |
Sex | Mean ± SEM PMI (hr) |
Median Braak Staging Scores | |
|---|---|---|---|---|
| NC | 87.0 ± 2.3 | 2M/6W | 3.0 ± 0.2 | I |
| PCAD | 85.0 ± 1.8 | 2M/6W | 2.8 ± 0.3 | IV* |
| MCI | 90.1 ± 2.1 | 3M/5W | 2.6 ± 0.2 | III* |
| LAD | 78.7 ± 2.1 | 2M/5W | 3.9 ± 0.9 | VI* |
p < 0.05
Figure 1.
A. Representative Western blot showing levels of EAAT-2 from NC, PCAD, MCI and LAD HPG. 1B. Representative Western blot of HHE-positive EAAT-2 immunoprecipitated from the same specimens. 1C. Western blot showing minimal immunostaining for the a negative control immunoprecipitation.
Figure 2.
Quantification of levels of total EAAT-2, and HHE-positive EAAT-2 expressed as mean ± SEM % control immunostaining. The figure also shows results for normalization of HHE-positive EAAT-2 to total EAAT-2 expressed as mean ± SEM% control. There was a significant (p < 0.05) increase in HHE levels for EAAT-2 immunoprecipitated from MCI and LAD hippocampus/parahippocampal gyrus. Note levels of HHE immunostaining are similar for PCAD, MCI and LAD specimens suggesting HHE modifications occur early in disease progression.
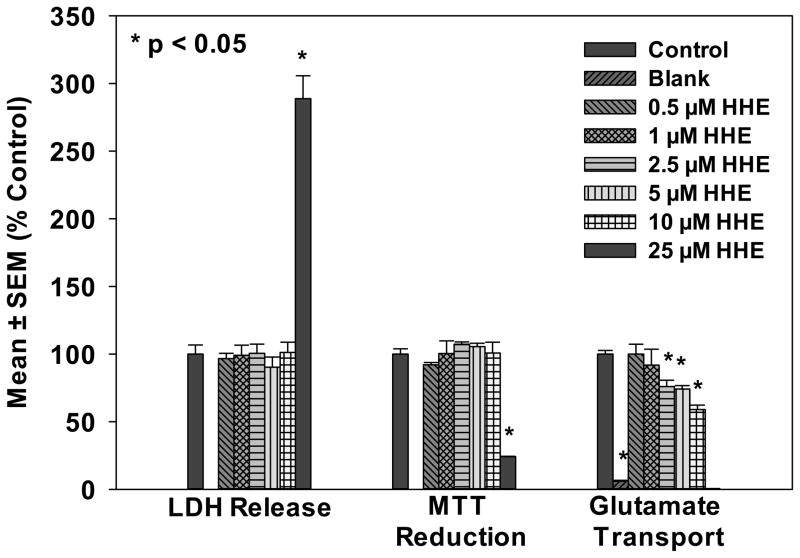
Treatment of primary rat astrocytes with HHE concentrations from 0.5 to 25 μM led to significantly increased LDH release and significantly decreased MTT reduction only at high (25 μM) concentrations (Figure 3). Although HHE treatment led to minimal astrocyte toxicity, it significantly decreased glutamate transport beginning at 2.5 μM HHE (Figure 3).
Figure 3.
Measurements of cell viability using MTT reduction (mitochondrial viability) and LDH release into the medium and levels of glutamate uptake in primary rat astrocytes. Results are expressed as mean ± SEM % control. Note that although low concentrations of HHE were not toxic as measured by LDH release into culture medium or MTT reduction concentrations beginning at 2.5 μM led to a significant decrease in glutamate uptake.
Discussion
Our previous studies showed levels of extractable HHE, a by-product of peroxidation of DHA, are significantly elevated in the HPG of PCAD and LAD subjects compared to age-matched NC subjects and that protein bound HHE is significantly elevated in the HPG of PCAD, MCI and LAD subjects compared to age-matched NC subjects [13]. In addition, previous studies from our laboratory [13] and others [28] showed HHE treatment of primary rat cortical neurons led to decreased cell viability. We also showed HHE treatment of primary rat cortical neurons led to a significant decrease in glucose uptake [22]. Because of the reactivity of α, β unsaturated aldehydes like HHE toward a variety of proteins, the present study was carried out to assess the effects of HHE on glutamate transport and to determine if EAAT-2, the main glutamate transporter in brain, showed significant HHE modification in the progression of AD. Our data show HHE was relatively well tolerated by primary astrocytes (2 weeks-in-culture) with no significant decrease in MTT reduction or increase in LDH release up to 25 μM HHE. In contrast, primary astrocytes showed significant decreases in glutamate uptake beginning at HHE concentrations of 2.5 μM suggesting a particular sensitivity of glutamate transporters to HHE. Immunoprecipitation of EAAT-2, the major glutamate transporter in brain, from HPG showed increased HHE reactivity associated with EAAT-2 from MCI and LAD subjects compared to NC subjects suggesting post translational modification of EAAT-2 occurs early and may contribute to the diminished glutamate transport observed in AD.
Although the exact pathogenesis of AD remains unclear, alterations in glutamate transport have been proposed as a mechanism by which synapses and neurons are injured [40]. Glutamate, the major excitatory amino acid of the central nervous system is critical for function of hippocampal and cortical pyramidal neurons and is involved in higher mental function, cognition, learning and memory [41]. Multiple studies show glutamate transport is altered in AD temporal and frontal cortices and hippocampus, blood lymphocytes, platelets and fibroblasts [42, 43] and in cultured astrocytes from AD patients and transgenic mouse models of amyloid deposition [44–47]. In addition, alterations in glutamate uptake in autopsy specimens are well correlated with multiple indices of cell death and with synapse loss as measured by levels of synaptophysin and are thought to occur early in disease progression before the onset of cognitive decline [48].
Excessive stimulation of neurons by glutamate (excitotoxicity) is prevented by a family of five Na-dependent high affinity excitatory amino acid transporters (EAATs) which include GLAST (EAAT-1), EAAT-2 (or glutamate transporter-1; GLT-1), EAAT-3, EAAT-4 and EAAT-5. EAAT-1 and 2 and neuron specific EAAT-3 are the primary glutamate transporters in the hippocampus and neocortex [49] whereas EAAT-4 is mainly localized in the Bregman area of cerebellum [50, 51] and EAAT-5 is predominately associated with retina [52]. EAAT-2, the main glutamate transporter in forebrain is localized primarily in fine astrocytic processes throughout presynaptic and extrasynaptic regions [53, 54] and functions to transport glutamate into astrocytes maintaining basal extracellular concentrations in the low nM range [55] and preventing glutamate related injury to synapses.
Sutdies of EAAT-2 in AD show a significant decrease of protein in LAD brain [56–58] as well as significant reductions of EAAT-2 mRNA in AD frontal cortex without a corresponding decrease in EAAT-1 or EAAT-3 mRNA [59]. Other studies of LAD and NC HPG show decreased EAAT-1 and EAAT-2 mRNA with altered localization of proteins in NFT [57]. More recent studies of patients with a variety of tauopathies including AD, progressive supranuclear palsy and corticobasal degeneration show EAAT-2 preferentially interacts with hyperphosphorylated tau characteristic of neurofibrillary tangles [60]. In contrast, [61, 62] showed no significant changes of EAAT-2 in AD. Additional studies show EAAT-2 may be oxidatively modified by exposure to Aβ [25, 35, 63–66] or aldehydic byproducts of lipid peroxidation including 4-HNE and acrolein [23, 35] resulting in impaired glutamate transport. Indeed, recent studies using sequential solubilization of proteins from frontal cortex specimens of 100 clinically and pathologically characterized NC, MCI and LAD subjects demonstrated changes in EAAT-2 solubilization in MCI and LAD brain suggesting post translational modifications that led to the formation of high molecular weight, less soluble oligomers [49]. Previous studies show Aβ significantly impairs glutamate uptake in primary astrocytes [67, 68] as well as in synaptosomes prepared from mice [64, 66, 69]. In addition, HNE is shown to impair glutamate uptake by AD fibroblast cultures that was prevented by 24 h pretreatment with antioxidants (1 mM glutathione ethyl ester) [70]. Our current data suggest glutamate transport by primary astrocytes is significantly impaired by HHE. It should be noted that HHE at concentrations of 2.5 μM led to a significant decrease (40%) in glutamate transport whereas higher concentrations of acrolein, a related α, β-unsaturated aldehydic by-product of lipid peroxidation [23] were required for similar effects suggesting glutamate transporters may be particularly susceptible to modification by HHE. In addition, immuoprecipitation of EAAT-2 from specimens of HPG obtained from NC subjects and subjects through the progression of AD showed significant HHE modification of the protein in MCI, and LAD brain with levels in early disease (MCI, PCAD) comparable to those observed in late-stage disease (LAD) suggesting diminished ability of a key regulatory protein early in disease progression that might contribute to the neurodegeneration observed in AD. Although EAAT2 seems to be particularly sensitive to HHE exposure it is not the only protein that demonstrates HHE immunostaining. Our previous studies using SDS-PAGE and proteomics [13] identified multiple proteins that are HHE modified in LAD brain consistent with the broad reactivity of α, β-unsaturated aldehydes with lysinse, cysteine and histidine residues. Based on the broad reactivity of HHE with multiple proteins, use of DHA supplementation as a therapeutic approach should probably be in conjunction with antioxidants to minimize the negative effects of generation of HHE.
Overall, our data suggest HHE modification of EAAT-2 is an early event in the pathogenesis of AD and that HHE modification significantly decreases glutamate transport. Post translational modification of HHE could lead to loss of glutamate regulation and excitotoxic injury to synapses and neurons and may play a role in the pathogenesis of AD.
Acknowledgments
This research was supported by NIH grants 5P01-AG05119 and P30-AG028383. The authors thank the UK-ADC Neuropathology Core for subject diagnoses and Ms. Sonya Anderson for subject demographic data.
References
- 1.Lovell MA, Markesbery WR. Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer’s disease. Nucleic Acids Res. 2007;35:7497–7504. doi: 10.1093/nar/gkm821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 3.Nunomura A, Perry G, Hirai K, Aliev G, Takeda A, Chiba S, Smith MA. Neuronal RNA oxidation in Alzheimer’s disease and Down’s syndrome. Ann N Y Acad Sci. 1999;893:362–364. doi: 10.1111/j.1749-6632.1999.tb07855.x. [DOI] [PubMed] [Google Scholar]
- 4.Shan X, Tashiro H, Lin CL. The identification and characterization of oxidized RNAs in Alzheimer’s disease. J Neurosci. 2003;23:4913–4921. doi: 10.1523/JNEUROSCI.23-12-04913.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sultana R, Butterfield DA. Role of Oxidative Stress in the Progression of Alzheimer’s Disease. J Alzheimers Dis. 2009 doi: 10.3233/JAD-2010-1222. [DOI] [PubMed] [Google Scholar]
- 6.McGarth LT, McGleenon BM, Brennan S, McColl D, McIlroy S, Passmore AP. Increased oxidative stress in Alzheimer’s disease as assessed with 4-hydroxynonenal but not malondialdehyde. Q J Med. 2001;94:385–490. doi: 10.1093/qjmed/94.9.485. [DOI] [PubMed] [Google Scholar]
- 7.Lovell MA, Xie C, Markesbery WR. Acrolein is increased in Alzheimer’s disease brain and is toxic to primary hippocampal cultures. Neurobiol Aging. 2001;22:187–194. doi: 10.1016/s0197-4580(00)00235-9. [DOI] [PubMed] [Google Scholar]
- 8.Markesbery WR, Lovell MA. Four-hydroxynonenal, a product of lipid peroxidation, is increased in the brain in Alzheimer’s disease. Neurobiol Aging. 1998;19:33–36. doi: 10.1016/s0197-4580(98)00009-8. [DOI] [PubMed] [Google Scholar]
- 9.Williams TI, Lynn BC, Markesbery WR, Lovell MA. Increased levels of 4-hydroxynonenal and acrolein, neurotoxic markers of lipid peroxidation, in the brain in Mild Cognitive Impairment and early Alzheimer’s disease. Neurobiol Aging. 2006;27:1094–1099. doi: 10.1016/j.neurobiolaging.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Aluise CD, Robinson RA, Beckett TL, Murphy MP, Cai J, Pierce WM, Markesbery WR, Butterfield DA. Preclinical Alzheimer disease: brain oxidative stress, Abeta peptide and proteomics. Neurobiol Dis. 2010;39:221–228. doi: 10.1016/j.nbd.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aluise CD, Robinson RA, Cai J, Pierce WM, Markesbery WR, Butterfield DA. Redox proteomics analysis of brains from subjects with amnestic mild cognitive impairment compared to brains from subjects with preclinical Alzheimer disease: Insights into memory loss in MCI. J Alzheimers Dis. 2011;23:257–269. doi: 10.3233/JAD-2010-101083. [DOI] [PubMed] [Google Scholar]
- 12.Bradley MA, Markesbery WR, Lovell MA. Increased levels of 4-hydroxynonenal and acrolein in the brain in preclinical Alzheimer disease. Free Radic Biol Med. 2010;48:1570–1576. doi: 10.1016/j.freeradbiomed.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradley MA, Xiong-Fister S, Markesbery WR, Lovell MA. Elevated 4-hydroxyhexenal in Alzheimer’s disease (AD) progression. Neurobiol Aging. 2012;33:1034–1044. doi: 10.1016/j.neurobiolaging.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montine TJ, Montine KS, McMahan W, Markesbery WR, Quinn JF, Morrow JD. F2-isoprostanes in Alzheimer and other neurodegenerative diseases. Antioxid Redox Signal. 2005;7:269–275. doi: 10.1089/ars.2005.7.269. [DOI] [PubMed] [Google Scholar]
- 15.Catala A. Lipid peroxidation of membrane phopholipids generates hydroxy-alkenals and oxidized phopholipids active in physiological and/or pathological conditions. Chemistry and Physics of Lipids. 2009;157:1–11. doi: 10.1016/j.chemphyslip.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Van Kuijk FJ, Holte LL, Dratz EA. 4-hydroxhexenal: a lipid peroxidation product derived from oxidized docosahexaenoic acid. Biochim Biophys Acta. 1990;1043:116–118. doi: 10.1016/0005-2760(90)90118-h. [DOI] [PubMed] [Google Scholar]
- 17.LoPachin RM, Barber DS, Gavin T. Molecular mechanisms of the conjugated alpha,beta-unsaturated carbonyl derivatives: relevance to neurotoxicity and neurodegenerative diseases. Toxicol Sci. 2008;104:235–249. doi: 10.1093/toxsci/kfm301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LoPachin RM, Gavin T, Petersen DR, Barber DS. Molecular mechanisms of 4-hydroxy-2-nonenal and acrolein toxicity: nucleophilic targets and adduct formation. Chem Res Toxicol. 2009;22:1499–1508. doi: 10.1021/tx900147g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lovell MA, Xie C, Markesbery WR. Acrolein is increased in Alzheimer’s disease brain and is toxic to primary hippocampal cultures. Neurobiology of Aging. 2001;22:187–194. doi: 10.1016/s0197-4580(00)00235-9. [DOI] [PubMed] [Google Scholar]
- 20.Montine KS, Olson SJ, Amarnath V, Whetsell WO, Jr, Graham DG, Montine TJ. Immunohistochemical detection of 4-hydroxy-2-nonenal adducts in Alzheimer’s disease is associated with inheritance of APOE4. Am J Pathol. 1997;150:437–443. [PMC free article] [PubMed] [Google Scholar]
- 21.Lovell MA, Ehmann WD, Mattson MP, Markesbery WR. Elevated 4-hydroxynonenal in ventricular fluid in Alzheimer’s disease. Neurobiol Aging. 1997;18:457–461. doi: 10.1016/s0197-4580(97)00108-5. [DOI] [PubMed] [Google Scholar]
- 22.Bradley MA, Xiong-Fister S, Markesbery WR, Lovell MA. Elevated 4-hydroxyhexenal in Alzheimer’s disease (AD) progression. Neurobiology of aging. 2012;33:1034–1044. doi: 10.1016/j.neurobiolaging.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lovell MA, Xie C, Markesbery WR. Acrolein, a product of lipid peroxidation, inhibits glucose and glutamate uptake in primary neuronal cultures. Free Radic Biol Med. 2000;29:714–720. doi: 10.1016/s0891-5849(00)00346-4. [DOI] [PubMed] [Google Scholar]
- 24.Mark RJ, Lovell MA, Markesbery WR, Uchida K, Mattson MP. A role for 4-hydroxynonenal, an aldehydic product of lipid peroxidation, in disruption of ion homeostasis and neuronal death induced by amyloid beta-peptide. J Neurochem. 1997;68:255–264. doi: 10.1046/j.1471-4159.1997.68010255.x. [DOI] [PubMed] [Google Scholar]
- 25.Keller JN, Germeyer A, Begley JG, Mattson MP. 17Beta-estradiol attenuates oxidative impairment of synaptic Na+/K+-ATPase activity, glucose transport, and glutamate transport induced by amyloid beta-peptide and iron. J Neurosci Res. 1997;50:522–530. doi: 10.1002/(SICI)1097-4547(19971115)50:4<522::AID-JNR3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 26.Pocernich CB, Butterfield DA. Acrolein inhibits NADH-linked mitochondrial enzyme activity: implications for Alzheimer’s disease. Neurotoxicity Research. 2003;5:515–520. doi: 10.1007/BF03033161. [DOI] [PubMed] [Google Scholar]
- 27.Mattson MP, Fu W, Waeg G, Uchida K. 4-Hydroxynonenal, a product of lipid peroxidation, inhibits dephosphorylation of the microtubule-associated protein tau. Neuroreport. 1997;8:2275–2281. doi: 10.1097/00001756-199707070-00036. [DOI] [PubMed] [Google Scholar]
- 28.Long EK, Murphy TC, Leiphon LJ, Watt J, Morrow JD, Milne GL, Howard JR, Picklo MJ., Sr Trans-4-hydroxy-2-hexenal is a neurotoxic product of docosahexaenoic (22:6; n-3) acid oxidation. Journal of neurochemistry. 2008;105:714–724. doi: 10.1111/j.1471-4159.2007.05175.x. [DOI] [PubMed] [Google Scholar]
- 29.Long EK, Rosenberger TA, Picklo MJ., Sr Ethanol withdrawal increases glutathione adducts of 4-hydroxy-2-hexenal but not 4-hydroxyl-2-nonenal in the rat cerebral cortex. Free radical biology & medicine. 2010;48:384–390. doi: 10.1016/j.freeradbiomed.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 30.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 31.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 32.Markesbery WR, Schmitt FA, Kryscio RJ, Davis DG, Smith CD, Wekstein DR. Neuropathologic substrate of mild cognitive impairment. Arch Neurol. 2006;63:38–46. doi: 10.1001/archneur.63.1.38. [DOI] [PubMed] [Google Scholar]
- 33.National Institute on Aging and the Reagan Institute Working on Group Diagnostic Cirteria for the Neuropathological Assessment of Alzheimer’s disease. Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. Neurobiology of Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- 34.Wiltfang J, Esselmann H, Bibl M, Smirnov A, Otto M, Paul S, Schmidt B, Klafki HW, Maler M, Dyrks T, Bienert M, Beyermann M, Ruther E, Kornhuber J. Highly conserved and disease-specific patterns of carboxyterminally truncated Abeta peptides 1-37/38/39 in addition to 1-40/42 in Alzheimer’s disease and in patients with chronic neuroinflammation. J Neurochem. 2002;81:481–496. doi: 10.1046/j.1471-4159.2002.00818.x. [DOI] [PubMed] [Google Scholar]
- 35.Keller JN, Mark RJ, Bruce AJ, Blanc E, Rothstein JD, Uchida K, Waeg G, Mattson MP. 4-Hydroxynonenal, an aldehydic product of membrane lipid peroxidation, impairs glutamate transport and mitochondrial function in synaptosomes. Neuroscience. 1997;80:685–696. doi: 10.1016/s0306-4522(97)00065-1. [DOI] [PubMed] [Google Scholar]
- 36.Koh JY, Choi DW. Quantitative determination of glutamate mediated cortical neuronal injury in cell culture by lactate dehydrogenase efflux assay. J Neurosci Methods. 1987;20:83–90. doi: 10.1016/0165-0270(87)90041-0. [DOI] [PubMed] [Google Scholar]
- 37.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 38.Swanson RA. Astrocyte glutamate uptake during chemical hypoxia in vitro. Neurosci Lett. 1992;147:143–146. doi: 10.1016/0304-3940(92)90580-z. [DOI] [PubMed] [Google Scholar]
- 39.Leonova J, Thorlin T, Aberg ND, Eriksson PS, Ronnback L, Hansson E. Endothelin-1 decreases glutamate uptake in primary cultured rat astrocytes. American journal of physiology. Cell physiology. 2001;281:C1495–1503. doi: 10.1152/ajpcell.2001.281.5.C1495. [DOI] [PubMed] [Google Scholar]
- 40.Lipton SA. Pathologically activated therapeutics for neuroprotection. Nat Rev Neurosci. 2007;8:803–808. doi: 10.1038/nrn2229. [DOI] [PubMed] [Google Scholar]
- 41.Kashani A, Lepicard E, Poirel O, Videau C, David JP, Fallet-Bianco C, Simon A, Delacourte A, Giros B, Epelbaum J, Betancur C, El Mestikawy S. Loss of VGLUT1 and VGLUT2 in the prefrontal cortex is correlated with cognitive decline in Alzheimer disease. Neurobiol Aging. 2008;29:1619–1630. doi: 10.1016/j.neurobiolaging.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 42.Ferrarese C, Begni B, Canevari C, Zoia C, Piolti R, Frigo M, Appollonio I, Frattola L. Glutamate uptake is decreased in platelets from Alzheimer’s disease patients. Ann Neurol. 2000;47:641–643. [PubMed] [Google Scholar]
- 43.Ferrarese C, Sala G, Riva R, Begni B, Zoia C, Tremolizzo L, Galimberti G, Millul A, Bastone A, Mennini T, Balzarini C, Frattola L, Beghi E. Decreased platelet glutamate uptake in patients with amyotrophic lateral sclerosis. Neurology. 2001;56:270–272. doi: 10.1212/wnl.56.2.270. [DOI] [PubMed] [Google Scholar]
- 44.Beart PM, O’Shea RD. Transporters for L-glutamate: an update on their molecular pharmacology and pathological involvement. Br J Pharmacol. 2007;150:5–17. doi: 10.1038/sj.bjp.0706949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cross AJ, Slater P, Simpson M, Royston C, Deakin JF, Perry RH, Perry EK. Sodium dependent D-[3H]aspartate binding in cerebral cortex in patients with Alzheimer’s and Parkinson’s diseases. Neurosci Lett. 1987;79:213–217. doi: 10.1016/0304-3940(87)90699-9. [DOI] [PubMed] [Google Scholar]
- 46.Hardy J, Cowburn R, Barton A, Reynolds G, Lofdahl E, O’Carroll AM, Wester P, Winblad B. Region-specific loss of glutamate innervation in Alzheimer’s disease. Neurosci Lett. 1987;73:77–80. doi: 10.1016/0304-3940(87)90034-6. [DOI] [PubMed] [Google Scholar]
- 47.Liang Z, Valla J, Sefidvash-Hockley S, Rogers J, Li R. Effects of estrogen treatment on glutamate uptake in cultured human astrocytes derived from cortex of Alzheimer’s disease patients. J Neurochem. 2002;80:807–814. doi: 10.1046/j.0022-3042.2002.00779.x. [DOI] [PubMed] [Google Scholar]
- 48.Scott HA, Gebhardt FM, Mitrovic AD, Vandenberg RJ, Dodd PR. Glutamate transporter variants reduce glutamate uptake in Alzheimer’s disease. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 49.Woltjer RL, Duerson K, Fullmer JM, Mookherjee P, Ryan AM, Montine TJ, Kaye JA, Quinn JF, Silbert L, Erten-Lyons D, Leverenz JB, Bird TD, Pow DV, Tanaka K, Watson GS, Cook DG. Aberrant detergent-insoluble excitatory amino acid transporter 2 accumulates in Alzheimer disease. J Neuropathol Exp Neurol. 2010;69:667–676. doi: 10.1097/NEN.0b013e3181e24adb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- 51.Kanai Y, Smith CP, Hediger MA. A new family of neurotransmitter transporters: the high-affinity glutamate transporters. FASEB J. 1993;7:1450–1459. doi: 10.1096/fasebj.7.15.7903261. [DOI] [PubMed] [Google Scholar]
- 52.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 53.Furness DN, Dehnes Y, Akhtar AQ, Rossi DJ, Hamann M, Grutle NJ, Gundersen V, Holmseth S, Lehre KP, Ullensvang K, Wojewodzic M, Zhou Y, Attwell D, Danbolt NC. A quantitative assessment of glutamate uptake into hippocampal synaptic terminals and astrocytes: new insights into a neuronal role for excitatory amino acid transporter 2 (EAAT2) Neuroscience. 2008;157:80–94. doi: 10.1016/j.neuroscience.2008.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lehre KP, Danbolt NC. The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. J Neurosci. 1998;18:8751–8757. doi: 10.1523/JNEUROSCI.18-21-08751.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Herman MA, Jahr CE. Extracellular glutamate concentration in hippocampal slice. J Neurosci. 2007;27:9736–9741. doi: 10.1523/JNEUROSCI.3009-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abdul HM, Sama MA, Furman JL, Mathis DM, Beckett TL, Weidner AM, Patel ES, Baig I, Murphy MP, LeVine H, 3rd, Kraner SD, Norris CM. Cognitive decline in Alzheimer’s disease is associated with selective changes in calcineurin/NFAT signaling. J Neurosci. 2009;29:12957–12969. doi: 10.1523/JNEUROSCI.1064-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jacob CP, Koutsilieri E, Bartl J, Neuen-Jacob E, Arzberger T, Zander N, Ravid R, Roggendorf W, Riederer P, Grunblatt E. Alterations in expression of glutamatergic transporters and receptors in sporadic Alzheimer’s disease. J Alzheimers Dis. 2007;11:97–116. doi: 10.3233/jad-2007-11113. [DOI] [PubMed] [Google Scholar]
- 58.Masliah E, Alford M, DeTeresa R, Mallory M, Hansen L. Deficient glutamate transport is associated with neurodegeneration in Alzheimer’s disease. Ann Neurol. 1996;40:759–766. doi: 10.1002/ana.410400512. [DOI] [PubMed] [Google Scholar]
- 59.Li S, Mallory M, Alford M, Tanaka S, Masliah E. Glutamate transporter alterations in Alzheimer disease are possibly associated with abnormal APP expression. J Neuropathol Exp Neurol. 1997;56:901–911. doi: 10.1097/00005072-199708000-00008. [DOI] [PubMed] [Google Scholar]
- 60.Sasaki K, Shimura H, Itaya M, Tanaka R, Mori H, Mizuno Y, Kosik KS, Tanaka S, Hattori N. Excitatory amino acid transporter 2 associates with phosphorylated tau and is localized in neurofibrillary tangles of tauopathic brains. FEBS Lett. 2009;583:2194–2200. doi: 10.1016/j.febslet.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 61.Beckstrom H, Julsrud L, Haugeto O, Dewar D, Graham DI, Lehre KP, Storm-Mathisen J, Danbolt NC. Interindividual differences in the levels of the glutamate transporters GLAST and GLT, but no clear correlation with Alzheimer’s disease. J Neurosci Res. 1999;55:218–229. doi: 10.1002/(SICI)1097-4547(19990115)55:2<218::AID-JNR9>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 62.Maragakis NJ, Dykes-Hoberg M, Rothstein JD. Altered expression of the glutamate transporter EAAT2b in neurological disease. Ann Neurol. 2004;55:469–477. doi: 10.1002/ana.20003. [DOI] [PubMed] [Google Scholar]
- 63.Guo Z, Ersoz A, Butterfield DA, Mattson MP. Beneficial effects of dietary restriction on cerebral cortical synaptic terminals: preservation of glucose and glutamate transport and mitochondrial function after exposure to amyloid beta-peptide, iron, and 3-nitropropionic acid. J Neurochem. 2000;75:314–320. doi: 10.1046/j.1471-4159.2000.0750314.x. [DOI] [PubMed] [Google Scholar]
- 64.Guo ZH, Mattson MP. Neurotrophic factors protect cortical synaptic terminals against amyloid and oxidative stress-induced impairment of glucose transport, glutamate transport and mitochondrial function. Cereb Cortex. 2000;10:50–57. doi: 10.1093/cercor/10.1.50. [DOI] [PubMed] [Google Scholar]
- 65.Keller JN, Pang Z, Geddes JW, Begley JG, Germeyer A, Waeg G, Mattson MP. Impairment of glucose and glutamate transport and induction of mitochondrial oxidative stress and dysfunction in synaptosomes by amyloid beta-peptide: role of the lipid peroxidation product 4-hydroxynonenal. J Neurochem. 1997;69:273–284. doi: 10.1046/j.1471-4159.1997.69010273.x. [DOI] [PubMed] [Google Scholar]
- 66.Lauderback CM, Hackett JM, Huang FF, Keller JN, Szweda LI, Markesbery WR, Butterfield DA. The glial glutamate transporter, GLT-1, is oxidatively modified by 4-hydroxy-2-nonenal in the Alzheimer’s disease brain: the role of Abeta1–42. J Neurochem. 2001;78:413–416. doi: 10.1046/j.1471-4159.2001.00451.x. [DOI] [PubMed] [Google Scholar]
- 67.Harris ME, Carney JM, Cole PS, Hensley K, Howard BJ, Martin L, Bummer P, Wang Y, Pedigo NW, Jr, Butterfield DA. beta-Amyloid peptide-derived, oxygen-dependent free radicals inhibit glutamate uptake in cultured astrocytes: implications for Alzheimer’s disease. Neuroreport. 1995;6:1875–1879. doi: 10.1097/00001756-199510020-00013. [DOI] [PubMed] [Google Scholar]
- 68.Harris ME, Wang Y, Pedigo NW, Jr, Hensley K, Butterfield DA, Carney JM. Amyloid beta peptide (25–35) inhibits Na+-dependent glutamate uptake in rat hippocampal astrocyte cultures. J Neurochem. 1996;67:277–286. doi: 10.1046/j.1471-4159.1996.67010277.x. [DOI] [PubMed] [Google Scholar]
- 69.Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Begni B, Brighina L, Sirtori E, Fumagalli L, Andreoni S, Beretta S, Oster T, Malaplate-Armand C, Isella V, Appollonio I, Ferrarese C. Oxidative stress impairs glutamate uptake in fibroblasts from patients with Alzheimer’s disease. Free Radic Biol Med. 2004;37:892–901. doi: 10.1016/j.freeradbiomed.2004.05.028. [DOI] [PubMed] [Google Scholar]