Abstract
Purpose
To determine the conversion to resectability in patients with unresectable liver metastases from colorectal cancer treated with hepatic arterial infusion (HAI) plus systemic oxaliplatin and irinotecan (CPT-11).
Patients and Methods
Forty-nine patients with unresectable liver metastases (53% previously treated with chemotherapy) were enrolled onto a phase I protocol with HAI floxuridine and dexamethasone plus systemic chemotherapy with oxaliplatin and irinotecan.
Results
Ninety-two percent of the 49 patients had complete (8%) or partial (84%) response, and 23 (47%) of the 49 patients were able to undergo resection in a group of patients with extensive disease (73% with > five liver lesions, 98% with bilobar disease, 86% with ≥ six segments involved). For chemotherapy-naïve and previously treated patients, the median survival from the start of HAI therapy was 50.8 and 35 months, respectively. The only baseline variable significantly associated with a higher resection rate was female sex. Variables reflecting extensive anatomic disease, such as number of lesions or number of vessels involved, were not significantly associated with the probability of resection.
Conclusion
The combination of regional HAI floxuridine/dexamethasone and systemic oxaliplatin and irinotecan is an effective regimen for the treatment of patients with unresectable liver metastases from colorectal cancer, demonstrating a 47% conversion to resection (57% in chemotherapy-naïve patients). Future randomized trials should compare HAI plus systemic chemotherapy with systemic therapy alone to assess the additional value of HAI therapy in converting patients with hepatic metastases to resectability.
INTRODUCTION
Primary resection of liver metastases from colorectal carcinoma is potentially curative, with a 10-year survival of 20%.1,2 A majority of patients who achieve a complete clinical response after systemic chemotherapy harbor residual disease.3,4 Therefore, hepatic resection has been advocated as the standard of care for patients who have resectable hepatic metastases from colorectal carcinoma. Despite advances in surgical technique, only 10% to 20% of patients with colorectal carcinoma are candidates for resection of hepatic metastases.5,6
Neoadjuvant chemotherapy may result in significant downstaging of liver metastases, allowing for one-stage7,8 or two-stage resections.9 The correlation of response rate and resection rate is strong (r = 0.96, P = .002) in patients with confined liver metastases.10 Resectability has been proposed as a novel end point for trials evaluating neoadjuvant chemotherapy for metastatic liver disease. In the study by Bismuth et al7 using infusional fluorouracil, leucovorin, and oxaliplatin in patients with unresectable hepatic metastases from colorectal carcinoma, 15% became resectable, with an increase in median survival for the patients who underwent resection. A treatment strategy to improve resection rate is regional chemotherapy using hepatic arterial infusion (HAI). The hepatic extraction rate with HAI of floxuridine is 95%,11,12 thus producing minimal systemic toxicity and allowing concomitant administration of systemic chemotherapy such as oxaliplatin and irinotecan at nearly full doses. An earlier report on HAI (floxuridine and dexamethasone) with systemic irinotecan and oxaliplatin demonstrated a median survival time of 36 months in a population of 22 previously treated patients with unresectable liver metastasis.13 This report examines the resectability rate in 49 patients treated with this regimen.
PATIENTS AND METHODS
Patient Selection
All patients had histologically confirmed colorectal adenocarcinoma with unresectable liver metastases. Reasons for initial unresectability are summarized under initial tumor involvement in Table 1. Most patients had lesions involving at least six segments in combination with involvement of major vessels (ie, hepatic vein, portal vein, and/or inferior vena cava), which would require resection of the vessel for an R0 resection. Tumors that involved all three main hepatic veins or both inflow pedicles were considered unresectable. Bilobar disease or number of metastases did not exclude a patient from consideration for resection, provided a complete resection was possible. All cases were reviewed at a multidisciplinary conference involving surgeons, radiologists, and medical oncologists, where patients were deemed unresectable.
Table 1.
Initial and Post-Treatment Characteristics of the 23 Patients, Though Initially Unresectable, Who Were Able to Undergo Liver Resection After HAI and Systemic Therapy
| Patient | Initial Tumor Involvement |
Post-Treatment Tumor Involvement |
||||||
|---|---|---|---|---|---|---|---|---|
| No. of Segments* | No. of HV† | No. of PV‡ | IVC | No. of Segments* | No. of HV† | No. of PV‡ | IVC | |
| 1 | 7 | 2 | 0 | Yes | 6 | 1 | 0 | 0 |
| 2 | 7 | 0 | 2 | Yes | 5 | 0 | 1 | 0 |
| 3 | 7 | 1 | 1 | Yes | 3 | 0 | 0 | 0 |
| 4 | 6 | 1 | 1 | 0 | 4 | 0 | 0 | 0 |
| 5 | 6 | 3 | 1 | 0 | 3 | 1 | 0 | 0 |
| 6 | 7 | 1 | 1 | 0 | 5 | 1 | 1 | 0 |
| 7 | 5 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8 | 6 | 1 | 0 | Yes | 5 | 0 | 0 | 0 |
| 9 | 7 | 2 | 2 | 0 | 5 | 1 | 0 | 0 |
| 10 | 7 | 3 | 1 | Yes | 5 | 2 | 0 | Yes |
| 11 | 7 | 3 | 0 | Yes | 4 | 2 | 0 | 0 |
| 12 | 4 | 2 | 0 | Yes | 2 | 2 | 0 | Yes |
| 13 | 8 | 3 | 2 | Yes | 7 | 2 | 0 | Yes |
| 14 | 7 | 1 | 2 | Yes | 5 | 1 | 1 | Yes |
| 15 | 5 | 1 | 1 | 0 | 5 | 1 | 0 | 0 |
| 16 | 8 | 3 | 1 | Yes | 7 | 2 | 0 | 0 |
| 17 | 8 | 2 | 1 | Yes | 3 | 1 | 1 | 0 |
| 18 | 8 | 1 | 1 | Yes | 6 | 0 | 1 | 0 |
| 19 | 7 | 1 | 0 | Yes | 6 | 0 | 0 | Yes |
| 20 | 6 | 0 | 0 | 0 | 4 | 0 | 0 | 0 |
| 21 | 8 | 2 | 1 | Yes | 3 | 0 | 0 | 0 |
| 22 | 7 | 1 | 2 | 0 | 5 | 1 | 1 | 0 |
| 23 | 5 | 2 | 0 | Yes | 5 | 2 | 0 | Yes |
NOTE. 0 = no.
Abbreviations: HAI, hepatic arterial infusion; HV, hepatic veins; PV, portal veins; IVC, inferior vena cava.
No. of hepatic segments involved by tumor.
No. of hepatic veins involved by tumor of the three main hepatic veins.
No. of main PV branches involved by tumor of the two principle branches (main right and left portal veins).
Patients were ineligible if they had evidence of extrahepatic disease, prior hepatic radiation, previous floxuridine treatment, symptomatic peripheral sensory neuropathy, or previous or concurrent malignancy (patients free of this disease for at least 5 years were considered eligible). Adequate organ function was required: WBC count ≥ 3,000 cells/μL, platelets ≥ 100,000 cells/μL, and serum total bilirubin level ≤ 2 mg/dL and a Karnofsky performance status ≥ 60%. If the primary tumor was present, it was removed when the pump was placed. Prior treatment for metastatic disease was permitted if the last dose had been administered at least 1 month before pump placement, but only two prior doses of oxaliplatin were allowed. Written informed consent was required, and the protocol was approved by the Memorial Sloan-Kettering Cancer Center institutional review board.
Pretreatment Evaluation, Pump Placement, and Follow-Up
Pretreatment evaluation included a complete history, physical examination, and laboratory studies including CBC, total bilirubin, alkaline phosphatase, carcinoembryonic antigen (CEA), lactate dehydrogenase (LDH), and AST obtained within 1 week before commencement of chemotherapy. Computed tomography (CT) scans of the chest, abdomen, and pelvis were obtained within 6 weeks before surgery, and to evaluate hepatic arterial supply, all patients underwent a preoperative hepatic CT angiogram that included visualization of the celiac and superior mesenteric arteries. Surgical guidelines for pump placement have been previously reported.14 Suspicious lymph nodes and/or extrahepatic masses were biopsied, and a cholecystectomy was performed. The pump catheter was positioned at the junction of the proper and common hepatic arteries, usually via the gastroduodenal artery. The distal gastroduodenal, the right gastric artery, and small branches supplying the stomach, duodenum, or pancreas were ligated, as were all accessory hepatic arteries. Intraoperative liver perfusion was tested. The catheter was secured in the artery with at least two nonabsorbable ties. Postoperatively, technetium macroaggregated human serum albumin was infused via the port of the pump to assess adequacy of perfusion. During treatment, all patients underwent evaluation of CBC and liver function tests every 2 weeks and CT of the chest, abdomen, and pelvis every 8 to 10 weeks. Responses were confirmed by the radiologists and then measured separately by a reference radiologist (L.S.). A complete response required the disappearance of all disease on CT and normalization of CEA. A partial response was defined as a reduction of ≥ 50% in the sum of the products of the greatest perpendicular diameters of tumor nodules measured on any follow-up CT compared with baseline. A reduction of less than 50% was considered stable disease.15CT scans were re-evaluated every 2 months for response, and if response was extensive, patients were presented at a multidisciplinary conference to determine whether resection was possible. Patients with enough response to allow a one-stage or planned two-stage hepatic resection with curative intent were taken to the operating room. Preoperative portal vein embolization and intraoperative radiofrequency ablation were performed at the discretion of the treating surgeon. Intraoperative hepatic ultrasound was performed on all patients.
An experienced hepatobiliary surgeon reviewed baseline and preresection CT scans and recorded which segments were involved and whether hepatic veins, portal veins, or the inferior vena cava were involved. The tumor extent was recorded in Table 1.
Chemotherapy Administration
Patients received systemic chemotherapy and HAI floxuridine/dexamethasone in one of two phase I treatment cohorts: a 4-week cycle or a 5-week cycle. In both treatment groups, chemotherapy was initiated 2 to 3 weeks after pump surgery. Patients in the 4-week cycle received both regional (via the HAI Codman pump; Johnson & Johnson, Piscataway, NJ) and systemic chemotherapy concurrently. Floxuridine was delivered in a 14-day infusion at 0.12 mg/kg × 30 divided by flow rate. Dexamethasone at 1 mg × kg × 30 divided by flow rate was always placed in the pump with floxuridine heparin and saline. On day 15, a 14-day infusion of heparin (30,000 units) and 30 mL of saline were administered via the pump. Systemic chemotherapy (oxaliplatin 85 to 100 mg/m2 and irinotecan 100 to 200 mg/m2) was administered on day 1 and 15 of each schedule. Patients on the 5-week cycle received HAI therapy on day 1 for a 14-day infusion and heparinized saline on days 15 and 29. Systemic chemotherapy with escalating doses of oxaliplatin at 85 to 100 mg/m2 and irinotecan at 100 to 150 mg/m2 was administered on days 15 and 29. Patients received protocol treatment until one of the following events occurred: a reduction in the extent of disease that rendered the patient a candidate for resection, hepatic or extrahepatic progression of disease, or excessive toxicity.
Study Design and Statistical Analysis
The two phase I treatment cohorts were pooled to analyze the clinical activity of a chemotherapy regimen of HAI floxuridine/dexamethasone and systemic irinotecan and oxaliplatin in patients with unresectable liver metastases. Twenty-two patients on the 4-week schedule have already been reported13 and are included in this analysis. Overall survival, time to hepatic progression, and progression-free survival were estimated using the Kaplan-Meier method. Survival curves were compared using the log-rank test. To identify potential predictive factors for resection, the association of different baseline clinical, laboratory, and radiologic variables with resection was examined. Clinical variables analyzed included age, sex, previous treatment, primary site, primary stage, and synchronous disease. Examined radiologic variables included number, size, and distribution of liver lesions; extent of disease (% liver involvement); and vascular involvement. Baseline CEA, alkaline phosphatase, bilirubin, LDH, and the clinical risk score2 were also evaluated as potential predictive factors for resectability. Associations of the different potential predictive factors with resectability were assessed using the Fisher's exact test for categoric variables and using the exact Wilcoxon rank sum test for continuous variables. A P value less than .05 was considered statistically significant. All statistical analyses were conducted in SAS 9.1.
RESULTS
Patient Characteristics
Patients with unresectable liver metastases from colorectal carcinoma were put on a “pending list” before HAI pump placement. Patients were registered to protocol only when all eligibility requirements were met. Of the 55 patients on the pending list, seven patients were excluded for the following reasons: three patients had complications from resection of the primary colorectal cancer (abscess or anastomotic leak), two patients could not have pumps placed because of hepatic artery stenosis, and two patients had extrahepatic perfusion on technetium-labeled microaggregated albumin scans. Therefore, 49 patients were registered to protocol and treated on one of the two treatment regimens: 22 patients on the 4-week cycle and 27 patients on the 5-week cycle. Patient characteristics for the 4- and 5-week groups are similar and are reported together (Table 2). Ninety percent of patients had a clinical risk score2 ≥ 3 (scores 1, 2, 3, 4, and 5, 2%, 8.2%, 38.8%, 40.8%, and 10.2%, respectively). Seventeen patients (35%) had their primary tumor resected at the time of pump placement.
Table 2.
Patient Characteristics: Univariate Analysis of Predictive Factors for Resection
| Categorical Variable | Total |
Resected |
Unresected |
P | ||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |||
| Resection status | 23 | 26 | ||||||
| Sex | ||||||||
| Male | 33 | 67 | 10 | 30 | 23 | 70 | .002 | |
| Female | 16 | 33 | 13 | 81 | 3 | 19 | ||
| Previous chemotherapy | ||||||||
| Pretreated | 26 | 53 | 10 | 38 | 16 | 62 | .26 | |
| Chemotherapy-naïve | 23 | 47 | 13 | 57 | 10 | 43 | ||
| Site of disease | ||||||||
| Colon | 42 | 86 | 21 | 50 | 21 | 50 | .42 | |
| Rectum | 7 | 14 | 2 | 29 | 5 | 71 | ||
| Dukes' stage | ||||||||
| B | 10 | 20 | 4 | 40 | 6 | 60 | .73 | |
| C | 39 | 80 | 19 | 49 | 20 | 51 | ||
| Time to liver metastases | ||||||||
| Synchronous | 36 | 73 | 18 | 50 | 18 | 50 | .53 | |
| Metachronous | 13 | 27 | 5 | 38 | 8 | 62 | ||
| Clinical risk score | ||||||||
| ≥ 3 | 44 | 90 | 20 | 45 | 24 | 55 | .75 | |
| 0-2 | 5 | 10 | 3 | 60 | 2 | 40 | ||
| Lesions | ||||||||
| > 5 | 36 | 73 | 18 | 50 | 18 | 50 | .64 | |
| 1-5 | 13 | 27 | 5 | 38 | 8 | 62 | ||
| Size > 5 cm | 20 | 41 | 10 | 50 | 10 | 50 | .78 | |
| Size ≤ 5 cm | 29 | 59 | 13 | 45 | 16 | 55 | ||
| Lobular involvement | ||||||||
| Bilobar | 48 | 98 | 23 | 48 | 25 | 52 | 1 | |
| Unilobar | 1 | 2 | 0 | 0 | 1 | 100 | ||
| Bordering vena cava | ||||||||
| Yes | 25 | 51 | 14 | 56 | 11 | 44 | .26 | |
| No | 24 | 49 | 9 | 38 | 15 | 63 | ||
| Liver involvement | ||||||||
| ≥ 50 | 17 | 35 | 7 | 41 | 10 | 59 | .76 | |
| < 50 | 32 | 65 | 16 | 50 | 16 | 50 | ||
| All segments involved | 12 | 24 | 5 | 42 | 7 | 58 | .75 | |
| All hepatic veins involved | 12 | 24 | 4 | 33 | 8 | 67 | .33 | |
| Both portal veins involved | 16 | 33 | 5 | 31 | 11 | 69 | .14 | |
| Age, years | ||||||||
| Median | 59 | 53.5 | 61.5 | .22 | ||||
| Range | 25-77 | 25-71 | 32-77 | |||||
| CEA, ng/ml | ||||||||
| Median | 109.5 | 75.5 | 229.3 | .08 | ||||
| Range | 3.2-5,340.4 | 3.2-1,308 | 5.7-5,340.4 | |||||
| LDH, Units/L | ||||||||
| Median | 247 | 263 | 215 | .57 | ||||
| Range | 94-1,021 | 94-1020 | 126-1,021 | |||||
Abbreviations: CEA, carcinoembryonic antigen; LDH, lactate dehydrogenase.
Dose-escalation and toxicity results have been reported for the 4-week cycle13; grade 3 to 4 toxicities during the first two cycles included diarrhea (14%) and neutropenia (23%). Toxicity after the first two cycles included neutropenia (18%), neurotoxicity (23%), and bilirubin more than 3 mg/mL (5%). Patients enrolled onto the 5-week cycle had the following toxicities during the first two cycles: grade 3 diarrhea (33%), grade 3 or 4 alkaline phosphatase (15% and 11%, respectively), grade 3 to 4 AST (19%), grade 3 bilirubin (4%), and grade 3 or 4 neutropenia (19% and 4%, respectively). Late toxicity (after the first two cycles) included grade 3 diarrhea (7%), grade 3 or 4 alkaline phosphatase (22% and 11%, respectively), grade 3 to 4 AST (7%), grade 3 or 4 neutropenia (15% and 11%, respectively), and neurotoxicity (19%).
Significant postoperative complications occurred in two patients (9%; hematoma and a noninfected fluid collection; both had interventional radiology drainage). Three patients required stent placements, 4 years, 3 years, and 8 months after study completion, and all had progression of disease before their stent placement.
Clinical Activity of HAI Floxuridine/Dexamethasone and Systemic Oxaliplatin and Irinotecan
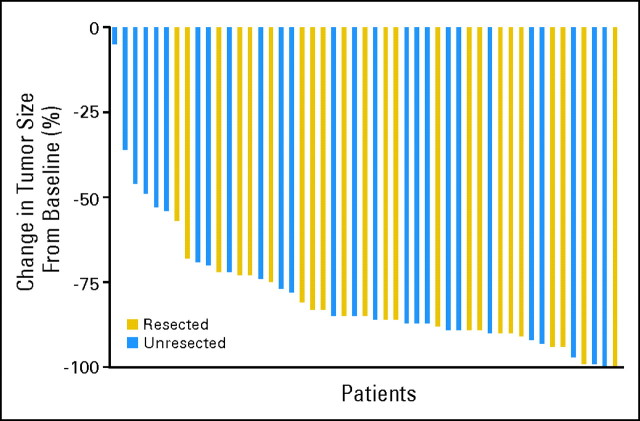
Forty-five (92%) of 49 patients had a complete or partial response: 84% had a partial response (Fig 1), and 8% had a complete response (waterfall graph, Fig 2). The resectability rate was 47%, and three patients had pathologic complete response.
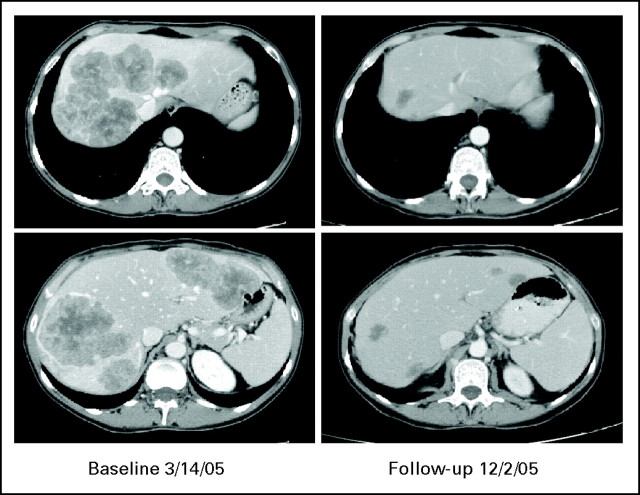
Fig 1.
A patient with extensive disease involving all vessels who, after response, was able to undergo resection. Carcinoembryonic antigen decreased from 156 to 2.9. Patient still has no recurrence 2 years after resection.
Fig 2.
Waterfall graph depicting the decrease in tumor measurements from baseline in patients treated with hepatic arterial infusion floxuridine/dexamethasone plus systemic oxaliplatin/irinotecan. −100 means 100% decrease in tumor. Blue bars represent patients who were not able to undergo resection. Gold bars represent patients who, though initially unresectable, after response with hepatic arterial infusion and systemic chemotherapy were able to undergo resection.
The median follow-up time from pump placement was 26 months, with a range of 8 to 78 months, and the median overall survival from pump placement was 39.8 months (95% CI, 33.7 to 50.9 months). For chemotherapy-naïve patients, the response rate was 100% (23 of 23 patients), median survival was 50.8 months (95% CI, 41.1 months to not reached; Fig 3), and the resection rate was 57%. For previously treated patients, response rate was 85% (22 of 26 patients), median survival was 35 months (95% CI, 25 to 41.4 months; log-rank P = .02), and resection rate was 38%.
Fig 3.
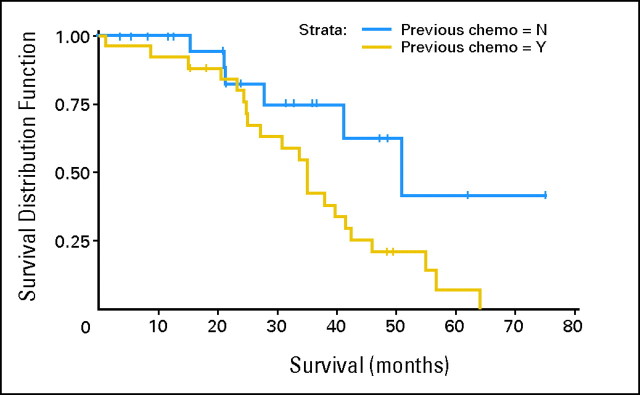
Median survival differences in patients who were either chemotherapy-naïve (50.8 months) or who had received previous systemic chemotherapy (35 months). Chemo, chemotherapy; N, no; Y, yes.
Characteristics of Resection
Twenty-three patients underwent resection (R0 in 19). Median time to resection was 7 months (range, 4 to 32 months). Twelve patients required portal vein embolization to increase the size of the future liver remnant. Four patients were considered for a planned two-stage resection, and 10 patients required radiofrequency ablation in addition to resection. Four patients had an extended right and one had an extended left resection. Hepatic parenchyma was normal in 28%, had focal congestion in 6%, and had mild to moderate steatosis in 67%. Median disease-free survival among the patients who underwent R0 resection was 7.6 months (95% CI, 5.2 to 12.9). All patients received systemic therapy after resection, and 13 patients received HAI therapy as well.
The characteristics of the patients who did not undergo resection include the following: 92% had six or more segments involved, and major vessel involvement included three hepatic veins (31%), two portal veins (42%), and vena cava (42%). Two patients with complete response did not undergo resection, and one is still free of disease for 3 years, whereas the other patient experienced disease recurrence in the liver 22 months after stopping treatment.
Predictive Factors for Resection
On univariate analysis, resection rates did not differ according to number, size, or distribution of tumors; disease-free interval; clinical risk score; or vascular involvement of liver metastases (Table 2). Neither the location of the primary tumor nor the prior chemotherapy history affected resectability. In contrast, patient sex was significantly associated with resectability (P = .002). The resection rate for male patients was 30%, as compared with 81% for female patients. The median age among patients who underwent resection was 54 years, as compared with 61.5 years among patients who did not undergo resection (P = .22). Sex remained significantly associated with resection (P = .006), even after adjusting in a logistic regression model for several risk factors, such as the clinical risk score (a summary of tumor-related characteristics), age, baseline CEA, and baseline LDH. The mean delivered doses of oxaliplatin, irinotecan, and floxuridine and the toxicity were examined between men and women, and there were no significant differences. The resectability rate for patients on the 4- and 5-week chemotherapy cycles were 36% and 56%, respectively.
Downstaging Required for Resection
For patients who became eligible for resection, clearance of several segments and vessels occurred as outlined in Table 1 under post-treatment tumor involvement. Some patients who presented with as many as eight segments and involvement of all three hepatic veins were able to undergo resection.
DISCUSSION
Criteria for unresectable hepatic metastases from colorectal carcinoma vary among trials of neoadjuvant therapy and complicate interpretation and comparison. Two major criteria apply: (1) true unresectable disease, signifying massive tumor extent that leads to inability to achieve a margin-negative resection that leaves at least two segments of liver behind with adequate arterial/portal inflow, venous outflow, and biliary drainage; (2) relative unresectable disease, such as high clinical risk score, which includes multiple hepatic metastases and synchronous disease. Under the second definition, the biology of the metastases puts into question the value of a hepatectomy, even if the disease is technically resectable. For true unresectable disease, downstaging is required. In contrast, for relative unresectable disease, even a regimen that produces only stable disease may encourage subsequent hepatic resection. For patients with identical criteria to define unresectable disease, surgical skills may determine different resectability rates. Therefore, a detailed description of reasons for initial unresectability and factors that allow subsequent resection should be delineated.
A common misconception is that only patients with limited tumor burden will become resectable after neoadjuvant chemotherapy, and thus patients with more extensive liver metastases may not be referred for multidisciplinary evaluation. The results of this analysis showed that even variables reflecting extensive anatomic disease (number of lesions or vessels involved) or variables associated with aggressive biologic behavior were not associated with a decreased probability of resection if patients were treated with HAI and systemic therapy.
In this trial, female sex led to a higher resection rate. In a Cancer and Leukemia Group B study using HAI versus systemic flourouracil/leucovorin,16 the survival was increased in the entire group receiving HAI (24 v 20 months), but in female patients, the survival difference was even greater (29.4 v 20 months for the HAI and systemic groups, respectively). In this study, male patients had significantly more biliary toxicity. In the present report, there was no significant difference in dose or toxicity between male and female patients.
A number of trials have looked at resection rates after systemic chemotherapy.8,17–20 Comparison of resection rates among these trials is difficult given nonuniform criteria to define unresectable disease, actual reasons for unresectability, and proportion of previously treated patients. Our results compare favorably, despite the fact that our population is characterized by a higher proportion of adverse characteristics, and more than half of our patients (53%) had been previously treated with systemic therapy (35% with prior fluorouracil, leucovorin, and irinotecan). Not surprisingly, median survival for chemotherapy-naïve patients (50.8 months) was significantly better compared with that of previously treated patients (Fig 3).
Detailed description of initial tumor extent and how disease became resectable is lacking in some trials, as seen by reports on patients with unresectable disease who were able to undergo resection after achieving stable disease. A detailed description of the initial tumor extent in this trial confirms that these patients truly had unresectable disease.
The high response rate seen in this study may be due to the use of three active agents. In a randomized phase III trial involving 244 patients, fluorouracil, oxaliplatin and irinotecan showed improved response rates (60% v 34%; P < .0001), progression-free survival (median 9.8 v 6.9 months; P = .0006), and overall survival (median 22.6 v 16.7 months), with worse toxicity (grade 3 to 4 neutropenia, 50% v 28%; P = .0006) compared with a standard fluorouracil, leucovorin, and irinotecan regimen.21 Among the 39 patients with liver-only metastases who were treated with the fluorouracil, oxaliplatin and irinotecan regimen, 36% were able to undergo resection. The patients had not been previously treated with irinotecan or oxaliplatin.
In conclusion, even patients with extensive hepatic metastases from colorectal carcinoma, whether previously treated or untreated with chemotherapy, may become resectable with combined therapy using HAI and systemic chemotherapy. To compare this study with other studies looking at preoperative chemotherapy in unresectable disease, it is vital to know the patient criteria used to determine unresectability. To evaluate whether HAI therapy increases resectability, a multi-institutional randomized trial is needed that compares the best systemic chemotherapy with or without HAI in patients with unresectable hepatic metastases from colorectal carcinoma.
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00695201.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: Nancy E. Kemeny, Pfizer, sanofi-aventis; Yuman Fong, sanofi-aventis; William R. Jarnagin, sanofi-aventis; Michael D'Angelica, sanofi-aventis Research Funding: Nancy E. Kemeny, Pfizer, sanofi-aventis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Nancy E. Kemeny, Fidel D. Huitzil Melendez, Marinela Capanu, Michael D'Angelica
Financial support: Nancy E. Kemeny
Administrative support: Nancy E. Kemeny
Provision of study materials or patients: Nancy E. Kemeny, Philip B. Paty, Yuman Fong, William R. Jarnagin, Michael D'Angelica
Collection and assembly of data: Nancy E. Kemeny, Fidel D. Huitzil Melendez, Marinela Capanu, Lawrence H. Schwartz, Dina Patel, Michael D'Angelica
Data analysis and interpretation: Nancy E. Kemeny, Fidel D. Huitzil Melendez, Marinela Capanu, Lawrence H. Schwartz, Michael D'Angelica
Manuscript writing: Nancy E. Kemeny, Fidel D. Huitzil Melendez, Marinela Capanu, Michael D'Angelica
Final approval of manuscript: Nancy E. Kemeny, Fidel D. Huitzil Melendez, Marinela Capanu, Philip B. Paty, Yuman Fong, Lawrence H. Schwartz, William R. Jarnagin, Dina Patel, Michael D'Angelica
REFERENCES
- 1.Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 2.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: Analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. discussion 318-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benoist S, Brouquet A, Penna C, et al. Complete response of colorectal liver metastases after chemotherapy: Does it mean cure? J Clin Oncol. 2006;24:3939–3945. doi: 10.1200/JCO.2006.05.8727. [DOI] [PubMed] [Google Scholar]
- 4.Adam R, et al. Complete pathologic response after preoperative chemotherapy for colorectal liver metastases: Myth or reality? J Clin Oncol. 2008;26:1635–1641. doi: 10.1200/JCO.2007.13.7471. [DOI] [PubMed] [Google Scholar]
- 5.Scheele J. Hepatectomy for liver metastases. Br J Surg. 1993;80:274–276. doi: 10.1002/bjs.1800800302. [DOI] [PubMed] [Google Scholar]
- 6.Jarnagin WR, Conlon K, Bodniewicz J, et al. A clinical scoring system predicts the yield of diagnostic laparoscopy in patients with potentially resectable hepatic colorectal metastases. Cancer. 2001;91:1121–1128. doi: 10.1002/1097-0142(20010315)91:6<1121::aid-cncr1108>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 7.Bismuth H, Adam R, Levi F. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 1996;224:509–520. doi: 10.1097/00000658-199610000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adam RA, Ariche E, Giachetti A, et al. Five-year survival following hepatic resection after neoadjuvant therapy for nonresectable colorectal. Ann Surg Oncol. 2001;8:347–353. doi: 10.1007/s10434-001-0347-3. [DOI] [PubMed] [Google Scholar]
- 9.Adam R, Laurent A, Azoulay D, et al. Two-stage hepatectomy: A planned strategy to treat irresectable liver tumors. Ann Surg. 2000;232:777–785. doi: 10.1097/00000658-200012000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folprecht G, Grothey A, Alberts S, et al. Neoadjuvant treatment of unresectable colorectal liver metastases: Correlation between tumour response and resection rates. Ann Oncol. 2005;16:1311–1319. doi: 10.1093/annonc/mdi246. [DOI] [PubMed] [Google Scholar]
- 11.Ensminger WD, Rosowsky A, Raso V. A clinical pharmacological evaluation of hepatic arterial infusions of 5-fluoro-2-deoxyuridine and 5-fluorouracil. Cancer Res. 1978;38:3784–3792. [PubMed] [Google Scholar]
- 12.Collins JM. Pharmacologic rationale for regional drug delivery. J Clin Oncol. 1984;2:498–504. doi: 10.1200/JCO.1984.2.5.498. [DOI] [PubMed] [Google Scholar]
- 13.Kemeny N, Jarnaqin W, Paty P, et al. Phase I trial of systemic oxaliplatin combination chemotherapy with hepatic arterial infusion in patients with unresectable liver metastases from colorectal cancer. J Clin Oncol. 2005;23:4888–4896. doi: 10.1200/JCO.2005.07.100. [DOI] [PubMed] [Google Scholar]
- 14.Kemeny N, Daly J, Reichman B, et al. Intrahepatic or systemic infusion of fluorodeoxyuridine in patients with liver metastases from colorectal carcinoma: A randomized trial. Ann Intern Med. 1987;107:459–465. doi: 10.7326/0003-4819-107-4-459. [DOI] [PubMed] [Google Scholar]
- 15.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 16.Kemeny NE, Niedzwiecki D, Hollis DR, et al. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: A randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481) J Clin Oncol. 2006;24:1395–1403. doi: 10.1200/JCO.2005.03.8166. [DOI] [PubMed] [Google Scholar]
- 17.Alberts SR, Horvath WL, Sternfeld WC, et al. Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver-only metastases from colorectal cancer: A North Central Cancer Treatment Group phase II study. J Clin Oncol. 2005;23:9243–9249. doi: 10.1200/JCO.2005.07.740. [DOI] [PubMed] [Google Scholar]
- 18.Pozzo CB, Cassano M, Quirino A, et al. Neoadjuvant treatment of unresectable liver disease with irinotecan and 5-fluorouracil plus folinic acid in colorectal cancer patients. Ann Oncol. 2004;15:933–939. doi: 10.1093/annonc/mdh217. [DOI] [PubMed] [Google Scholar]
- 19.Ho WM, Ma B, Mok T, et al. Liver resection after irinotecan, 5-fluorouracil, and folinic acid for patients with unresectable colorectal liver metastases: A multicenter phase II study by the Cancer Therapeutic Research Group. Med Oncol. 2005;22:303–312. doi: 10.1385/MO:22:3:303. [DOI] [PubMed] [Google Scholar]
- 20.Clavien PAS, Morse N, Selzner M, et al. Downstaging of hepatocellular carcinoma and liver metastases from colorectal cancer by selective intra-arterial chemotherapy. Surgery. 2002;131:433–442. doi: 10.1067/msy.2002.122374. [DOI] [PubMed] [Google Scholar]
- 21.Falcone A, Ricci S, Brunetti I, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: The Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25:1670–1676. doi: 10.1200/JCO.2006.09.0928. [DOI] [PubMed] [Google Scholar]