Abstract
In our previous studies, psychological stress enhanced operant escape responding of male and female rats. The stressors that produced hyperalgesia were physical restraint and social defeat. Another source of stress is pain, but effects of nociceptive stimulation on pain sensitivity of laboratory animals have not been documented using a stress paradigm. Reflex responses to nociceptive stimulation are reduced by concurrent stimulation at a remote site (termed diffuse noxious inhibitory control). However, the usual method of evaluating stress in laboratory animals is to test for effects after termination of the stressor. Accordingly, operant escape performance of male and female rats was evaluated during two successive trials involving nociceptive thermal stimulation. The intent was to determine whether nociceptive sensitivity differed on first trials and during pain-induced stress on second trials. Compared to a first trial of 44.5°C stimulation, escape responding increased during a second trial of 44.5°C stimulation (preceded by an escape trial of 10°C). Similarly, escape from cold (10°C) was enhanced when preceded by escapable 44.5°C stimulation. Thus, nociceptive stimulation enhanced subsequent escape from thermal stimulation, demonstrating facilitation of pain by pain. In contrast, an opposite profile of reflex suppression followed prior nociceptive stimulation. Reflex responses to 44.5°C were decreased following a 10°C escape trial, and reflex responses to 10°C were decreased following a 44.5°C escape trial .
Keywords: Pain, Stress, Operant escape, Thermal sensitivity, Pain affect
Introduction
Investigations of pain modulation in laboratory animals include numerous demonstrations of nociceptive reflex suppression that is described as analgesia and is attributed to descending inhibition from the brain stem. However, descending modulation from the brain stem can influence motor output (Holmqvist & Lundberg.A, 1959; Lai et al., 1989; Taylor et al., 1997; Zemlan et al., 1983). Also, ascending projections from the brain stem can alter sensory transmission (e.g., Svensson, 1987). Therefore, it is important to evaluate pain modulation with procedures which reveal cerebral encoding of pain and are not critically dependent upon motoneuronal excitability.
Reflex responses of a hindlimb and neuronal responses in lumbar spinal segments can be attenuated by concurrent nociceptive stimulation within cervical dermatomes (Villanueva and Le Bars, 1995). A common interpretation of this phenomenon, termed diffuse noxious inhibitory control (DNIC), is that pain is inhibitory to pain, but this requires mutual inhibition at both sites. Instead, DNIC may represent dominance of one sensation over another, depending on the relative intensity of stimulation at two sites or a cervical to lumbar hierarchy. Supposition that DNIC represents a generalized inhibition of pain by pain requires documentation of sensation magnitudes elicited by different intensities of stimulation at disparate sites, singly and simultaneously (Lautenbacher et al., 2007). In addition, simultaneous stimulation at two sites requires controls for divided attention (Staud et al., 2003), and non-reflexive tests of pain processing are needed.
The neuronal circuits responsible for reflex suppression by stress, inappropriately termed stress induced analgesia (SIA), include the limbic system, the hypothalamic-pituitary-adrenal axis and the brain stem (Herman et al., 1996), resulting in descending modulation of spinal sympathetic outflow, motor output and afferent input (Ford & Finn, 2008). Based on peripheral and spinal influences of stress (Khasar et al., 2008; Li et al., 1998; Mokha et al., 1986; Sandkuhler et al., 1987), it has been presumed that reflex tests appropriately reveal effects on nociception. However, restraint and social defeat stress enhance escape from nociceptive thermal stimulation (King et al., 2007; Marcinkiewcz et al., 2008). The combination of reflex suppression but increased pain sensitivity in laboratory animals is consistent with the clinical profile of stress effects on reflexes and pain (Ford and Finn, 2008; Nilsen et al., 2007; Temml et al., 2007; Vierck, 2006; Zautra et al., 2007).
If pain produces stress (see discussion) and stress increases pain, then pain should be facilitatory to pain. Such a positive feedback loop would not be evident with the DNIC paradigm or similar procedures that do not evaluate changes over time. Because the stress response develops slowly, the testing paradigm for the present study involved two sequential periods (trials) of thermal stimulation spanning 30 min. Nociceptive sensitivity during the first trial, which is likely to initiate a stress reaction, was compared with sensitivity during the second trial, when stress should be well developed. Previous experience with other methods of stress induction predicted that escape responding would be enhanced but reflex responding would be decreased during second trials, relative to first trials.
Methods
Three groups of Long-Evans hooded rats were behaviorally trained and then tested. Escape from thermal stimulation was evaluated with 18 females and 14 males. Lick/guard responding was evaluated with 12 females. All rats were maintained on a standard 12-hour light/dark cycle and had access to food and water ad libitum. All experimental procedures complied with ethical guidelines and standards established by the Institutional Animal Care & Use Committee at the University of Florida and conformed to National Institutes of Health guidelines for care and use of experimental animals.
Escape training and testing
The testing apparatus for escape consisted of a dark (0.5 foot candles) compartment with a thermally regulated floor plate (6 in wide, 8 in long) and a brightly lit (3200 foot candles) compartment (6 in wide, 6 in long) with a thermally neutral platform. The two compartments were separated by a partition with a 2.5 by 2.5 in. opening on one side. The animals could ambulate freely between compartments, choosing between thermal stimulation in the dark plate compartment and an aversive level of bright light in the platform compartment. The apparatus was ventilated with forced room air.
After a period of acclimatization to the testing apparatus in room lighting and without thermal stimulation, rats were trained to escape from nociceptive thermal stimulation. The training procedure involved two series of sessions with a gradual increase in magnitude of thermal stimulation. The first series was conducted without bright light over the platform, and heat and cold stimulation progressed on alternate days from 36°C to 44°C in 2°C increments and from 36°C to 20°C, 15°C, 10°C and then 5°C. The temperature sequences were then presented with the light on in the platform compartment.
Following completion of training, sessions of escape testing were conducted Monday - Friday. Each day, the animals were placed sequentially into two escape apparatuses situated side by side in a sound-isolated, dark room. A 15 min. first trial began when an animal was placed on the thermal plate in apparatus #1, and a 15 min. second trial began with placement on the thermal plate in apparatus #2. Occupancy of each escape platform was detected by electronic proximity detectors and was timed by proprietary software. Sequences of trials within days were 44.5°C then 10°C and 10°C then 44.5°C. Escape behavior was recorded during both daily trials, permitting comparisons of performance in response to 10°C as a first trial and as a second trial after 44.5°C and in response to 44.5°C as a first trial and as a second trial after 10°C. The sequences of cold then hot or hot then cold trials of nociceptive stimulation were used as a conservative approach to the possibility that thermoregulatory mechanisms would determine responses on the second trial. For example, if skin temperatures were the primary determinant of escape, a first trial of cold stimulation would decrease responding to a second trial of heat stimulation.
Lick/guard training and testing
A group of female rats was tested Monday-Friday in a sequence of escape and then L/G trials in adjacent apparatuses. Reflex responses to cold stimulation occur very rarely for 10°C, necessitating a temperature of 0.3°C for testing L/G responsivity to cold stimulation. The sequences of temperatures for escape and then L/G testing in 15 min. trials were: 0.3°C (escape) then 44.5°C (L/G) and 44.5°C (escape) then 0.3°C (L/G).
For a L/G trial, the animal was placed on a heated or cooled plate (6 in. by 9 in) in an enclosure. Reflex responding was recorded by an observer, using keystrokes to time episodes of licking and guarding with proprietary software. Licking is a stereotyped response involving flexion of either forelimb with fanning of the toes and licking of the ventral surface of the paw. Guarding involves protracted and exaggerated flexion of one hindlimb that is distinct from ambulation within the test enclosure.
Data analysis
Latencies to the first lick or guard for 0.3°C and 44.5°C are highly variable and do not represent behavior during an entire trial period. For this reason, analysis focused on the duration of licking and guarding. T-tests for dependent groups provided statistical confirmation of differences in lick or guard durations during second trials (after an escape trial) and first trials.
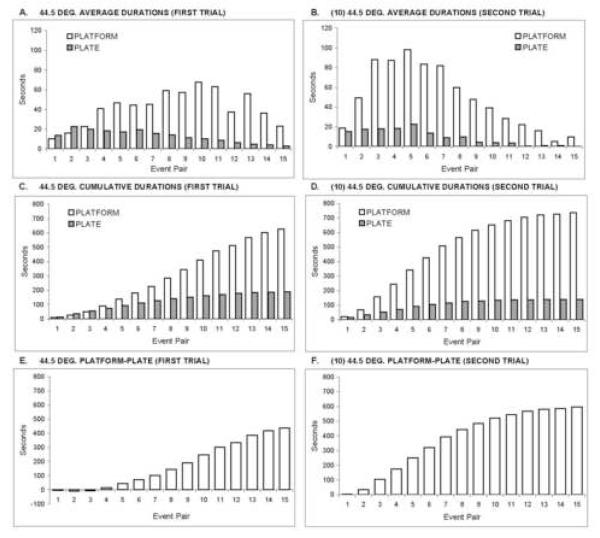
Our previous experiences with operant escape testing of rats have shown that first response latencies and the number of responses during a trial are poor indicants of nociceptive stimulus intensity. In contrast, the duration of platform occupancy (escape from thermal stimulation) reliably discriminates between thermal stimulus intensities and a variety of experimental manipulations presumed to affect pain intensity (Vierck et al., 2002, 2004; 2005; 2008a,c). In addition, the durations of successive occupancies of the plate and platform compartments provide important measures of changing sensitivity as a trial progresses. Therefore, as depicted in Figures 1 and 2, escape responding was broken down into individual occupancies of the plate and platform compartments. These discrete events occur in an alternating sequence as pairs of plate and platform durations (events).
Figure 1.
Operant escape performance of male rats during first trials at 44.5°C and second trials (following 10°C) at 44.5°C. (A) Average plate and platform durations for each of 15 sequential pairs of events (plate and platform durations) during first trials. (B) Average plate and platform durations during second trials. Plate durations gradually diminished as first and second trials progressed, and they did not differ between trials 1 and 2. Platform durations increased considerably as trial 1 progressed through event pair 11, and platform durations increased during trial 2, compared to trial 1. The decline in average plate and platform durations late in trials 1 and 2 results from fewer observations as the trials timed out for some animals. Therefore, the data are replotted as cumulative plate and platform durations across 15 event pairs during first trials (C) and second trials (D). The data are then plotted as platform minus plate durations, providing an event by event measure of pain sensitivity on first trials (E) and second trials (F).
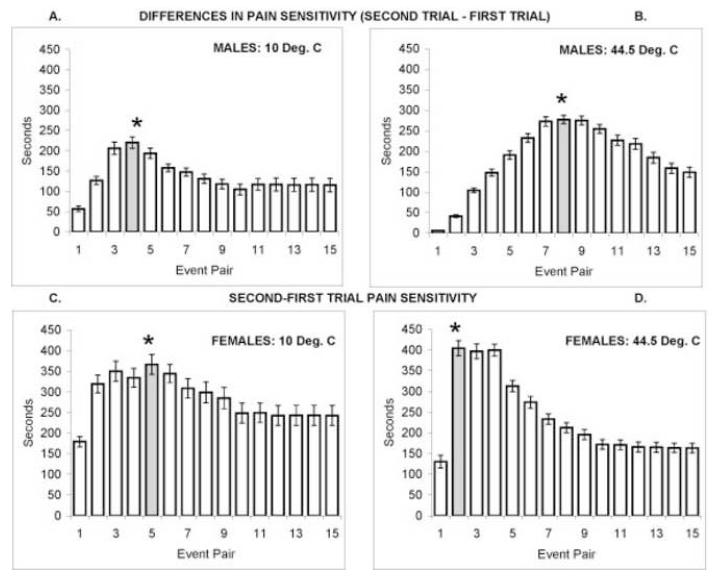
Figure 2.
Event by event effects of trial 1 on trial 2 are shown by subtracting the estimates of pain sensitivity in trial 1 (cumulative platform - plate durations, as in Figure 1E) from the estimates of pain sensitivity in trial 2 (as in Figure 1F). Positive values indicate that pain sensitivity was enhanced during second trials. Panels A (10°C) and B (44.5°C) show positive values throughout 15 event pairs for males. Panels C (10°C) and D (44.5°C) show positive values throughout 15 event pairs for females. T-tests for differences between trials 1 and 2 are indicated by * above the peak difference scores (shaded bars).
An experimental manipulation that produced hyperalgesia could decrease the duration of successive thermal stimuli endured (plate durations) or increase the duration of each escape response (platform durations). Because the number of these events can differ between animals in a time-limited session, durations were accumulated across 15 pairs of plate and platform occupancies. Summation of successive event durations for individual animals avoids problems associated with averaging across different numbers of observations late in a session. For a given test condition (e.g., first trials of 44.5°C stimulation) the accumulated durations of successive plate durations were subtracted from the accumulated durations of paired platform durations to give the relative preference for the escape platform or the thermal plate as a trial progressed (see Figure 1).
Increasingly positive platform minus plate durations indicate increasing pain sensitivity, and negative platform minus plate durations reveal relative insensitivity to nociceptive thermal stimulation. These difference scores were compared for first trials and second trials to indicate whether prior nociceptive stimulation increased or decreased pain sensitivity. Graphical displays of preference for the escape platform on first and second trials were used to determine the response number with the maximum difference in pain sensitivity (Figure 2). T-tests for dependent groups were then utilized to evaluate the significance of the maximal difference in pain sensitivity on first and second trials.
Results
Panels A and B of Figure 1 depict average durations of successive platform and plate occupancies of male rats during first and second trials of 44.5°C escape testing. Both panels reveal that the durations of thermal stimulation (on the plate) and escape (on the platform) changed over 15 pairs of these events. Early in first trials at 44.5°C (panel 1A), there was no preference for the thermal plate or escape platform (events 1-3), and the average occupancy within each compartment was short (10 to 20 sec). However, as trials progressed, plate times diminished slightly and platform times increased (responses 4-11). Average platform times eventually decreased, because the trial timed out for most of the animals (e.g. an animal with a maximum of 11 rather than 15 platform pairs). Platform times increased earlier and to a greater extent during second trials of 44.5°C that were preceded by a trial of 10°C (panel 1B).
Panels 1C and 1D accumulate successive platform and plate times for first and second trials respectively, depicting a progressive increase in difference between platform and plate durations. Also, second trial platform times (1D) increased faster than first trial platform times (1C), reflecting a greater sensitivity to thermal stimulation on second trials. Escape performance is further transformed into one curve showing cumulative differences (platform - plate times) for stimulation during first trials (1E) and second trials (1F). This expression of event-by-event differences in platform and plate times is useful for comparing thermal pain sensitivity during first and second trials (e.g., Figure 2B).
Figure 2 compares pain sensitivity for males and females responding to 10°C and 44.5°C during first and second trials. For each panel, event-by-event estimates of pain sensitivity (platform-plate durations) during first trials were subtracted from the corresponding calculations for second trials. Because of slightly reduced plate durations and substantially increased platform durations during second trials of 10°C and 44.5°C stimulation, pain sensitivity was greater for second trials compared to first trials (panels 2A-D). The uniformly positive values in Figure 2 show that pain sensitivity was increased throughout second trials of 10°C and 44.5°C stimulation for males and females. Statistical comparisons of maximal differences in pain sensitivity (Table 1) utilized t-tests for dependent samples of first and second trial platform minus plate durations for the event numbers shaded in Figure 2. All comparisons of platform-plate durations were significant, as were comparisons of platform durations. In contrast, plate durations for first and second trials did not differ significantly. Thus, latencies of escape (plate times) from cold or heat stimulation were not significantly reduced by prior nociceptive stimulation during first trials. The differences in pain sensitivity were expressed as a significant increase in escape duration (increased latency to return to thermal stimulation).
Table 1. Comparison of escape performance on first and second trials.
Statistical comparisons of escape behaviors on first and second trials for males and females during 10°C and 44.5°C stimulation. T-tests for dependent samples compared 3 measures of responsivity for the event pair with the maximal difference in pain sensitivity on first and second trials (see Figure 2). For males and females responding to 10°C and 44.5°C, platform durations were significantly increased during second trials, as were platform - plate durations. Plate durations on first and second trials were not significantly different.
| Platfrom-plate | Platform | Plate | |||||
|---|---|---|---|---|---|---|---|
| t | p | t | p | t | p | ||
| Males | 10 Deg. | 4.47 | <0.001 | 4.76 | <0.001 | 0.25 | 0.81 |
| 44.5 Deg. | 8.78 | <0.001 | 9.64 | <0.001 | 1.62 | 0.13 | |
| Females | 10 Deg. | 6.15 | <0.001 | 5.81 | <0.001 | 1.02 | 0.32 |
| 44.5 Deg. | 4.23 | <0.001 | 6.2 | <0.001 | 0.62 | 0.54 | |
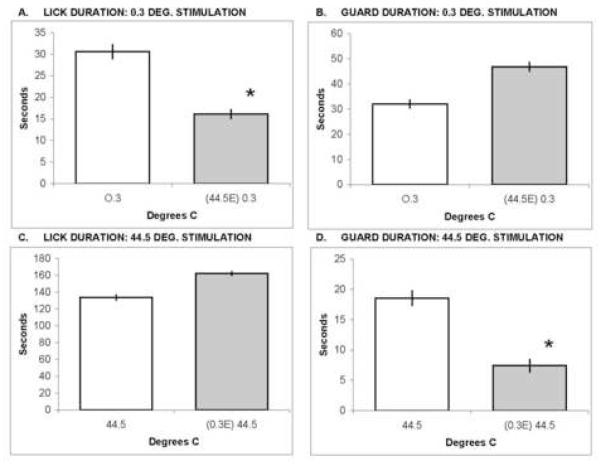
Figure 3 compares the duration of reflexive licking and guarding during first trials and during second trials after escape testing. Significant depression of lick duration was observed for second trials of 0.3°C stimulation following a first trial of 44.5°C escape testing (panel 3A; t=4.10, df=11, p=0.002). Also, the duration of guarding was significantly attenuated during second trials of 44.5°C stimulation after a first trial of 0.3°C escape testing (panel 3D; t=3.26, df=11, p<0.001). Reflex latencies on second trials were delayed but insignificantly, because of high variability (data not shown). Thus, attenuation of reflex responding by prior nociceptive stimulation was opposite to the facilitation observed with operant escape responding.
Figure 3.
Reflex response durations of female rats are shown for first and second trials at 0.3°C and 44.5°C . Second trials were preceded by an escape trial as denoted on the abscissas; e.g., in panel A: (44.5E) 0.3 indicates that the first trial was 44.5°C escape, and the data shown are from a second L/G trial at 0.3°C. Lick durations are shown in panels A and C; licking was significantly decreased (*) during second trials of 0.3°C stimulation, relative to first trials of 0.3°C stimulation. Guard durations are shown in panels B and D; guarding was significantly decreased (*) during second trials of 44.5°C, relative to first trials of 44.5°C stimulation.
Discussion
Painful experiences set in motion interrelated autonomic, emotional, sensory, motivational and motor effects that are recognized as components of a psychological stress response (Chapman et al., 2008; Craig, 2003). Stress can contribute to development of chronic pain and/or exacerbate it, once established (Vierck, 2006b). Although these mutually facilitatory relationships between pain and stress of humans are well known, preclinical studies using reflex tests have mostly emphasized the opposite relationship – concluding that pain and stress are inhibitory to pain. Numerous laboratory animal experiments have mapped out central pathways and transmitter systems responsible for reflex inhibition. Nociceptive input to any spinal level activates cells with projections to the periaqueductal gray and other nuclei of the brain stem (Craig, 2003) that send descending projections back to the spinal cord to modify nociceptive reflexes (Basbaum & Fields, 1979). In addition, nocireceptive spinal cells project to the limbic system (e.g., the amygdala) and the hypothalamus – important components of central stress circuits that act via brain stem nuclei to modulate reflex responses (Chapman et al., 2008). Effects on spinal transmission attributable to input from the brain stem include both inhibitory and facilitatory modulation (Porreca et al., 2002) of sensory transmission cells, autonomic nuclei and motoneurons (Millan, 2002; Lai et al., 1989).
Simple reflex responses have been utilized almost exclusively as behavioral tests of nociception revealing brain stem modulation of spinal output systems. An assumption implicit in this approach is that descending modulation is indiscriminately expressed, uniformly affecting motoneurons, autonomic output, input to reflex circuits, and sensory transmission to the brain. This is highly unlikely. In addition, reflexive behaviors that are present in spinal (Dimitrijevic & Nathan, 1967) or decerebrate (Woolf, 1984) animals cannot reveal effects of pain and stress on cerebral processing of nociception (Vierck, 2006a; Vierck et al., 2008b).
The present study supports the notion that nociceptive activation of brain stem circuits for descending modulation is inhibitory to nociceptive reflexes. However, the inhibition was specific for the two reflexes monitored (licking and guarding); it was not a generalized influence, even for reflexes. Following first trials of 44.5°C escape testing, licking was suppressed during second trials of cold stimulation (0.3°C), but guarding was not; and first trials of 0.3°C escape testing reduced guarding, but not licking, during second trials of heat stimulation (44.5°C). It is not clear that principles of motor control would predict this pattern of differential effects on licking and guarding, but descending control over a variety of reflex responses and motor actions has been shown to be specifically adapted to different environmental contingencies (e.g., Aggelopoulos et al., 1996; Schomburg, 1990; Taylor et al., 1997; Zemlan et al., 1983).
Stress can influence the sensitivity of peripheral nociceptors (Khasar et al., 2008) as a result of sympathetic activation (Craig, 2003) and release of adrenal hormones (Herman et al., 1996; Chapman et al., 2008), but if the only site of action were peripheral, both hyperalgesia and hypereflexia would be observed. Also, if descending control over spinal sensory transmission were a common mechanism for modulation of nociceptive reflexes and pain sensations, then a manipulation that reduces reflex responding to thermal stimulation would reduce escape responding to the same stimuli. However, second trial escape responding to cold (10°C) and heat (44.5°C) was increased following a first trial of nociceptive thermal stimulation, in contrast to decreased second trial reflex responding to cold and heat. If these effects resulted from brain stem modulation of spinal transmission, reflex circuits were inhibited and ascending spinal sensory projection systems were facilitated by prior nociceptive stimulation.
Further evidence for fundamental differences in neural processing within reflex circuits and pain pathways is provided by comparisons of latencies for reflex and escape responses to thermal stimulation. First latencies in response to 44°C on first trials were: 263.1 sec. for licking, 438.5 sec. for guarding and 16.6 sec. for escape. Reflex responses rarely occur for 10°C stimulation, but first escape latencies averaged 14.0 sec.; for 0.3°C stimulation, lick latencies were 240.6 sec. and guard latencies were 217.0 sec. Cold sensations and near threshold heat sensations became painful enough to escape long before reflex responses occurred. Therefore, it cannot be presumed that reflex latency, the usual measure of nociceptive sensitivity, represents the duration of stimulation required to elicit a pain sensation.
In the present study both escape latencies and escape durations were assessed repeatedly during trials of repetitive thermal stimulation. In contrast to many paradigms that predetermine the duration and rate of stimulation used to assess nociception, the animals determine both the duration and frequency of stimulation during escape testing. Plate durations correspond to escape latencies, representing the time that expires between the beginning of each thermal stimulus and escape. The measure of escape latency is often utilized in human studies of pain sensitivity, particularly involving long-duration stimulation (e.g., in cold pressor or tourniquet pain studies) which increases sensation intensity gradually over time before reaching a painful level. This applies to the present study, where low levels of aversive thermal stimulation were utilized, producing baseline escape latencies of approximately 15 sec. (see above). However, first trial nociceptive stimulation did not significantly affect escape latencies (plate durations) on second trials, indicating that pain thresholds were not increased. That is, escape occurred in response to similar skin temperatures and levels of nociceptor activation on both first and second trials.
Platform durations can be regarded as latencies of escape from bright light, but they differ greatly with variations in plate temperature (Vierck et al., 2002) while light intensity is a constant. Platform durations are determined primarily by the animals’ reaction to the plate temperature that drove them to the platform. Therefore, platform durations appear to represent affective responses of the animals following termination of each nociceptive stimulus on the plate. Reluctance to return to the plate is proportionate to the perceived intensity of the most recent exposure to nociceptive hot or cold stimulation. Platform duration is the measure that was significantly influenced by a prior trial of nociceptive stimulation. Platform durations for second trials of 44.5°C and 10°C were substantially increased by first trials of nociceptive stimulation.
There is little doubt that pain is stressful and, consistent with other stressors, sequential presentation of two trials of painful stimulation reveals enhancement of escape in the second trial, after stress is triggered in the first trial. The pattern of findings on second trials – increased escape and decreased reflex responding following first trials of nociceptive stimulation – is the same as that obtained following physical restraint or social defeat experiences that are regarded as stressful (King et al., 2003; Marcinkiewcz et al., 2008). Thus, in addition to short term mechanisms for central sensitization that enhance pain during repetitive activation of C nociceptors (Vierck et al., 1997), pain-induced stress extends the time-course of pain facilitation by pain.
Important functional interactions between stress and pain coding occur within cerebral structures and pathways that are distinct from brain stem and spinal reflex circuits. For example, anterior cingulate and prefrontal cortical regions have been implicated in affective processing of pain (Derbyshire & Jones, 1998; Craig, 2003; Johansen et al., 2001; Qiu et al., 2006) and evocation of stress reactions (Rauch et al., 2003; Radley et al., 2004). The increased affective reactions of rats to nociceptive stimulation on second escape trials likely resulted from interactions within these structures and related cerebral systems for stress and nociceptive processing.
Acknowledgments
Supported by the University of Florida Office of Research and the Comprehensive Center for Pain Research. Technical assistance of Karen Murphy is gratefully acknowledged.
References
- Aggelopoulos NC, Burton MJ, Clarke RW, Edgley SA. Characterization of a descending system that enables crossed group II inhibitory reflex pathways in the cat spinal cord. J Neurosci. 1996;16:723–729. doi: 10.1523/JNEUROSCI.16-02-00723.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. The origin of descending pathways in the dorsolateral funiculus of the spinal cord of the cat and rat: further studies on the anatomy of pain modulation. J Comp Neurol. 1979;187:513–531. doi: 10.1002/cne.901870304. [DOI] [PubMed] [Google Scholar]
- Chapman C, Tuckett R, Song C. Pain and stress in a systems perspective: reciprocal neural, endocrine and immune interactions. J Pain. 2008;9:122–145. doi: 10.1016/j.jpain.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A. Pain mechanisms: Labeled lines versus convergence in central processing. Ann Rev Neurosci. 2003;26:1–30. doi: 10.1146/annurev.neuro.26.041002.131022. [DOI] [PubMed] [Google Scholar]
- Derbyshire S, Jones A. Cerebral responses to a continual tonic pain stimulus measured using positron emission tomography. Pain. 1998;76:127–135. doi: 10.1016/s0304-3959(98)00034-7. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic MR, Nathan PW. Studies of spasticity in man. Brain. 1967;90:1–29. doi: 10.1093/brain/90.1.1. [DOI] [PubMed] [Google Scholar]
- Ford G, Finn D. Clinical correlates of stress-induced analgesia: Evidence from pharmacological studies. Pain. 2008;140:3–7. doi: 10.1016/j.pain.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Herman J, Prewitt C, Cullinan W. Neuronal circuit regulation of the hypothalamo-pituitary-adrenocortical stress axis. Crit Rev Neurobiol. 1996;10:371–394. doi: 10.1615/critrevneurobiol.v10.i3-4.50. [DOI] [PubMed] [Google Scholar]
- Holmqvist B, Lundberg A. On the organization of the supraspinal inhibitory control of interneurones of various spinal reflex arcs. Arch Ital Biol. 1959;97:340–356. [Google Scholar]
- Johansen J, Fields H, Manning B. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulated cortex. Proc Natl Acad Sci. 2001;98:8077–8082. doi: 10.1073/pnas.141218998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasar S, Burkham J, Dina O, Brown A, Bogen O, Alessandri-Haber N, Green P, Reichling D, Levine J. Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. J Neurosci. 2008;28:5721–5730. doi: 10.1523/JNEUROSCI.0256-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C, Devine D, Vierck CJ, Mauderli A, Yezierski R. Opioid modulation of reflex versus operant responses following stress in the rat. Neurosci. 2007;147:174–182. doi: 10.1016/j.neuroscience.2007.04.012. [DOI] [PubMed] [Google Scholar]
- King C, Rodgers J, Devine D, Vierck CJ, Yezierski R. Differential effects of stress on escape versus reflex responses to nociceptive thermal stimuli in the rat. Brain Res. 2003;987:214–222. doi: 10.1016/s0006-8993(03)03339-0. [DOI] [PubMed] [Google Scholar]
- Lai YY, Strahlendorf HK, Fung SJ, Barnes CD. The actions of two monoamines on spinal motoneurons from stimulation of the locus coeruleus in the cat. Brain Res. 1989;484:268–272. doi: 10.1016/0006-8993(89)90369-7. [DOI] [PubMed] [Google Scholar]
- Lautenbacher S, Prager M, Rollman G. Pain additivity, diffuse noxious inhibitory controls, and attention: a functional measurement analysis. Somatosens Mot Res. 2007;24:189–201. doi: 10.1080/08990220701637638. [DOI] [PubMed] [Google Scholar]
- Li HS, Monhemius R, Simpson BA, Roberts MH. Supraspinal inhibition of nociceptive dorsal horn neurones in the anaesthetized rat: tonic or dynamic? J Physiol. 1998;506:459–469. doi: 10.1111/j.1469-7793.1998.459bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkiewcz C, Green M, Devine D, Duarte P, Vierck CJ, Yezierski R. Social defeat stress potentiates thermal sensitivity in operant models of pain processing. Brain Research. 2008 doi: 10.1016/j.brainres.2008.11.042. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Progress in Neurobiology. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Mokha SS, McMillan JA, Iggo A. Pathways mediating descending control of spinal nociceptive transmission from the nuclei locus coeruleus (LC) and raphe magnus (NRM) in the cat. Exp Brain Res. 1986;61:597–606. doi: 10.1007/BF00237586. [DOI] [PubMed] [Google Scholar]
- Nilsen KB, Sand T, Westgaard RH, Stovner LJ, White LR, Leistad RB, Helde G, Ro M. Autonomic activation and pain in response to low-grade mental stress in fibromyalgia and shoulder/neck pain patients. Europ J Pain. 2007;11:743–755. doi: 10.1016/j.ejpain.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Porreca F, Ossipov M, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–326. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Noguchi Y, Honda M, Nakata H, Tamura Y, Tanaka S, Sadato H, Wang X, Inui K, Kakigi R. Brain processing of the signals ascending through unmyelinated C fibers in humans: An event-related functional magnetic resonance imaging study. Cereb Cortex. 2006;16:1289–1295. doi: 10.1093/cercor/bhj071. [DOI] [PubMed] [Google Scholar]
- Radley J, Sisti H, Hao J, Rocher A, McCall T, Hof P, McEwen B, Morrison J. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neurosci. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Rauch S, Shin E, Pitman R, Carson M, McMullin K, Whalen P, Makris N. Selectively reduced regional cortical volumes in post-traumatic stress disorder. Neuroreport. 2003;14:913–916. doi: 10.1097/01.wnr.0000071767.24455.10. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J, Fu QG, Zimmermann M. Spinal pathways mediating tonic or stimulation-produced descending inhibition from the periaqueductal gray or nucleus raphe magnus are separate in the cat. J Neurophysiol. 1987;58:327–341. doi: 10.1152/jn.1987.58.2.327. [DOI] [PubMed] [Google Scholar]
- Schomburg ED. Spinal functions in sensorimotor control of movements. Neurosurg Rev. 1990;13:179–185. doi: 10.1007/BF00313016. [DOI] [PubMed] [Google Scholar]
- Staud R, Robinson ME, Vierck CJ, Price DD. Diffuse noxious inhibitory controls (DNIC) attenuate temporal summation of second pain in normal males but not in normal females or fibromyalgia patients. Pain. 2003;101:167–174. doi: 10.1016/s0304-3959(02)00325-1. [DOI] [PubMed] [Google Scholar]
- Svensson T. Peripheral, autonomic regulation of locus coeruleus noradrenergic neurons in brain: putative implications for psychiatry and psychopharmacology. Psychopharmacol. 1987;92:1–7. doi: 10.1007/BF00215471. [DOI] [PubMed] [Google Scholar]
- Taylor J, Friedman R, Munson J, Vierck C. Stretch hyperreflexia of triceps surae muscles in the conscious cat after dorsolateral spinal lesions. J Neurosci. 1997;17:5004–5015. doi: 10.1523/JNEUROSCI.17-13-05004.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temml C, Wehrberger C, Riedl C, Ponholzer A, Marszalek M, Madersbacher S. Prevalence and correlates for instersititial cystitis symptoms in women participating in a health screening project. Europ Urol. 2007;51:803–809. doi: 10.1016/j.eururo.2006.08.028. [DOI] [PubMed] [Google Scholar]
- Vierck C. Animal models of Pain. In: McMahon S, Koltzenburg M, Aalfs CM, editors. Wall and Melzack’s Textbook of Pain. Elsevier, Churchill Livingston; London: 2006a. pp. 175–185. [Google Scholar]
- Vierck CJ. Mechanisms underlying development of spatially distributed chronic pain (fibromyalgia) Pain. 2006b;124:242–263. doi: 10.1016/j.pain.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Vierck CJ, Acosta-Rua A, Nelligan R, Tester N, Mauderli A. Low dose systemic morphine attenuates operant escape but facilitates innate reflex responses to thermal stimulation. J Pain. 2002;3:309–319. doi: 10.1054/jpai.2002.125186. [DOI] [PubMed] [Google Scholar]
- Vierck C, Acosta-Rua A, Johnson R. Bilateral chronic constriction of the sciatic nerve: A model of long-term cold hyperalgesia. J Pain. 2005;6:507. doi: 10.1016/j.jpain.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Vierck C, Acosta-Rua A, Rossi H, Neubert J. Sex differences in thermal pain sensitivity and sympathetic reactivity for two strains of rat. 2008a;9:739–749. doi: 10.1016/j.jpain.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierck CJ, Cannon RL, Fry G, Maixner WL. Characteristics of temporal summation of second pain sensations elicited by brief contact of glabrous skin by a preheated thermode. J Neurophysiol. 1997;78:992–1002. doi: 10.1152/jn.1997.78.2.992. [DOI] [PubMed] [Google Scholar]
- Vierck C, Hansson P, Yezierski R. Clinical and pre-clinical pain assessment: Are we measuring the same thing? Pain. 2008b;135:7–10. doi: 10.1016/j.pain.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Vierck C, Kline R, IV, Wiley R. Comparison of operant escape and innate reflex responses to nociceptive skin temperatures produced by heat and cold stimulation of rats. Behav Neurosci. 2004;118:627–635. doi: 10.1037/0735-7044.118.3.627. [DOI] [PubMed] [Google Scholar]
- Vierck C, Yezierski R, Light A. Long-lasting hyperalgesia and sympathetic dysregulation after formalin injection into the rat hind paw. Neurosci. 2008c;153:501–508. doi: 10.1016/j.neuroscience.2008.02.027. [DOI] [PubMed] [Google Scholar]
- Villanueva L, Le Bars D. The activation of bulbo-spinal controls by peripheral nociceptive inputs: diffuse noxious inhibitory controls. Biol Res. 1995;28:113–125. [PubMed] [Google Scholar]
- Woolf CJ. Long term alterations in the excitability of the flexion reflex produced by peripheral tissue injury in the chronic decerebrate rat. Pain. 1984;18:325–343. doi: 10.1016/0304-3959(84)90045-9. [DOI] [PubMed] [Google Scholar]
- Zautra AJ, Parrish BP, Puymbroeck CMV, Tennen H, Davis MC, Reich JW, Irwin M. Depression history, stress, and pain in rheumatoid arthritis patients. J Behav Med. 2007;30:187–197. doi: 10.1007/s10865-007-9097-4. [DOI] [PubMed] [Google Scholar]
- Zemlan FP, Kow LM, Pfaff DW. Effect of interruption of bulbospinal pathways on lordosis, posture, and locomotion. Exp Neurol. 1983;81:177–194. doi: 10.1016/0014-4886(83)90167-x. [DOI] [PubMed] [Google Scholar]