Fig. 1.
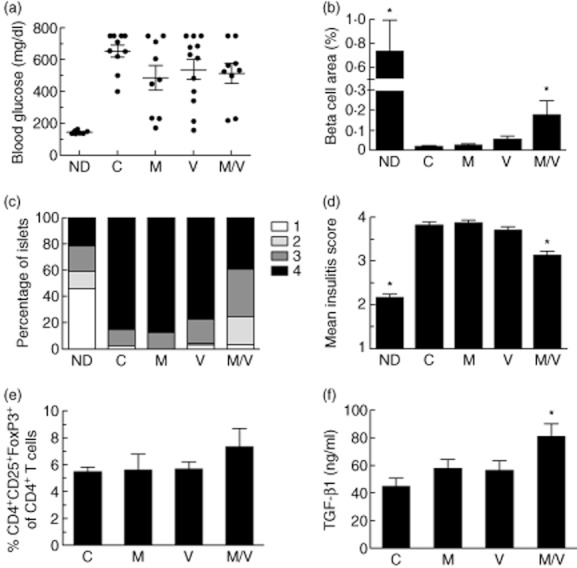
Effects of 4 weeks of single or combination therapy on non-obese diabetic (NOD) mice; 12–16-week-old diabetic female NOD mice were implanted with subcutaneous insulin-releasing pellets and randomized to drug treatments for 4 weeks (C = vehicle controls; M = MK-626; V = vorinostat; M/V = MK-626 and vorinostat) and compared to 22-week-old non-diabetic (ND) control NOD mice (n = 10 mice per group). (a) Random blood glucose levels at the end of the treatment period after removal of insulin pellets; (b) results of β cell area as a percentage of total pancreatic area in mice from each treatment group at the end of the treatment period (n = 4–9 mice per group); (c) insulitis scores at the end of the treatment period as a percentage of total islets for each treatment group (≥100 islets were scored from a total of four to nine mice per group); (d) mean insulitis score for each treatment group; (e) percentage of CD4+CD25+forkhead box protein 3 (Foxp3+) cells among total CD4+ lymphocytes in pancreatic lymph nodes for each treatment group at the end of the treatment period (n = 3–8 mice per group); (f) serum transforming growth factor (TGF)-β1 levels for each treatment group at the end of the treatment period (n = 4–6 per group). *P < 0·05 compared to vehicle controls in all panels.
