Fig. 1.
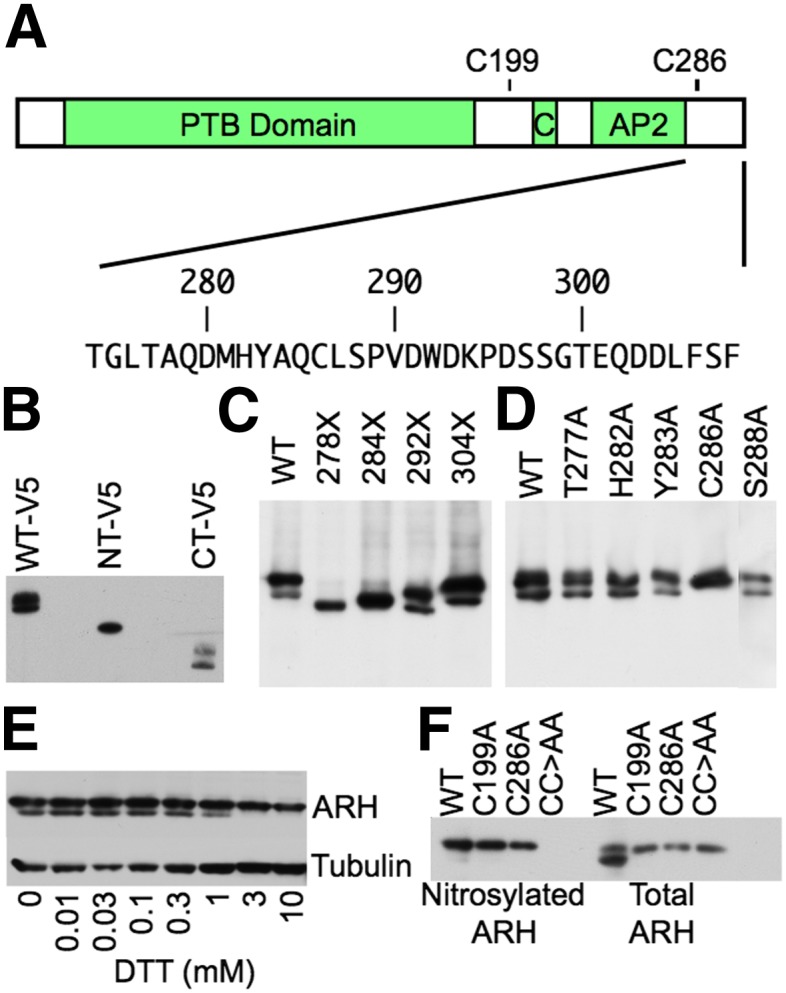
ARH is nitrosylated at C199 and C286. (A) Domain structure of ARH. ARH has a PTB domain (PTB), Clathrin Box module (C), and an AP-2-binding module (AP2). Below the domain structure is the amino acid sequence of the C-terminal 44 amino acids. (B) Identification of a posttranslational modification in the C-terminal half of ARH. HEK293 cells were transfected with C-terminal V5-tagged human ARH constructs encompassing the entire ARH protein (WT-V5), residues 1–187 (NT-V5), or residues 188–308 (CT-V5), and then lysed. Lysates were run on SDS-PAGE and immunoblotted for V5. (C) Posttranslational modification resides between residues 284 and 292. HEK293 cells were transfected with untagged human ARH variants bearing premature stop codons at the indicated residue positions and lysed. Lysates were run on SDS-PAGE and immunoblotted for ARH. (D) C286 is posttranslationally modified. HEK293 cells were transfected with human ARH variants bearing alanine mutations at the indicated positions and lysed. Lysates were run on SDS-PAGE and immunoblotted for ARH. (E) C286 participates in an intramolecular disulfide bond. Lysates of normal human fibroblasts were treated with the indicated concentrations of DTT for 30 min at room temperature, run on SDS-PAGE, and immunoblotted for ARH. (F) C286 forms a disulfide bridge with C199, and both C199 and C286 are nitrosylated. The C-terminal 121 residues of human ARH have two cysteines, C199 and C286. HEK293 cells were transfected with the indicated ARH variants and lysed. Lysates were divided into two portions. The first portion was processed by biotin switch to label nitrosylated cysteines with biotin. Biotinylated proteins were precipitated with neutravidin agarose and run with the second, untreated portion of the lysate on SDS-PAGE and immunoblotted for ARH. CC>AA indicates the ARH variant with both the C199A and C286A mutations.
