Figure 3.
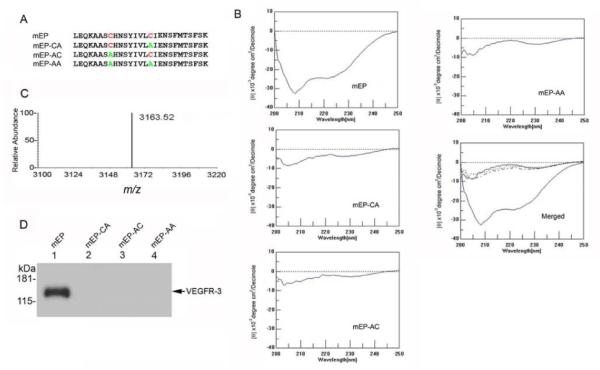
Substitutions within the mEP cysteine loop abolish binding to recombinant VEGFR-3. (A) Sequences of synthetic peptides containing Cys-to-Ala substitutions as indicated (B) Analysis of endostatin peptides using circular dichroism. The pronounced trough between −205 nm and −225 nm indicates secondary structure in the mEP peptide, whereas the substitution peptides mEP-CA, -AC and –AA lack this feature. (C) MALDI-TOF of mEP showed a single peak with a m/z ratio of 3163.52. (D) Protein pull-down assays with 50 μg mEP (lane 1), mEP-CA (lane 2), mEP-AC (lane 3), or mEP-AA (lane 4) found binding to recombinant VEGFR-3 by mEP via western blot using anti-VEGFR-3 antibody.
