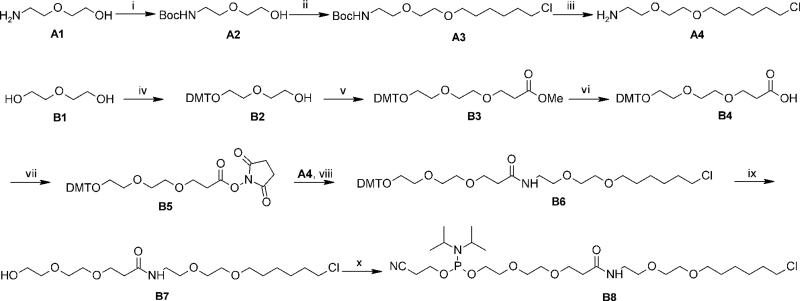
Scheme 1.
Reagents and conditions: i) Boc2O, anhyd. EtOH, 0 °C to rt., 2 h, 99%. ii) NaH, 6-chloro-1-iodohexane, THF/DMF, 0 °C to rt, o/n, 69%. iii) TFA, CH2Cl2, 0 °C to rt, 2 h, 81%. iv) DMT-Cl, Et3N, CH2Cl2, rt, 6 h, 67 %. v) Methyl acrylate, NaH, THF, 0 °C, 1 h, 75 %. vi) LiOH, MeOH/H2O, rt, 2h, 93 %. vii) N-hydroxysuccinimide, DCC, CH2Cl2, 0 °C to rt, o/n, 95%. viii) DIPEA, CH2Cl2, rt, o/n, 82%. ix) AcOH, H2O, rt, 2h, 68%. ×) DIPEA, 2-cyanoethyl N,N-diisopropylchlorophosphoramidite, CH2Cl2, 0 °C, 45 min, 98%.