Figure 2.
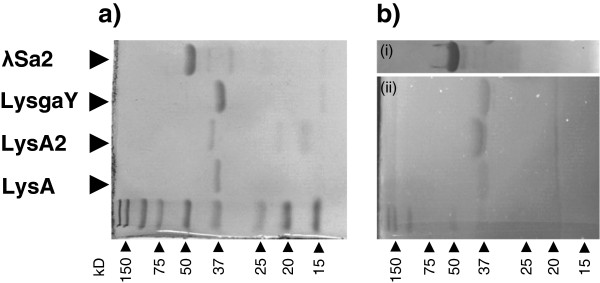
SDS-PAGE and exolytic activity analyses of Immobilized Metal Affinity Chromatography purified recombinant lysins. a) SDS-PAGE with high intensity bands correspond to predicted molecular weights for lysins λSa2 endolysin (51.9 kD), LysgaY (33.9 kD), LysA2 (37.4 kD), and LysA (36.4 kD). b) Zymogram analysis with whole cells substrate (i) Lactobacillus reuteri isolate 14171 and (ii) Lactobacillus amylovorus isolate 4540, co-polymerized within the polyacrylamide gel. Lysin exolytic activity resulted in visible clearing (dark bands) of the cell substrate at the point of protein localization, which corresponds with predicted molecular weights.
