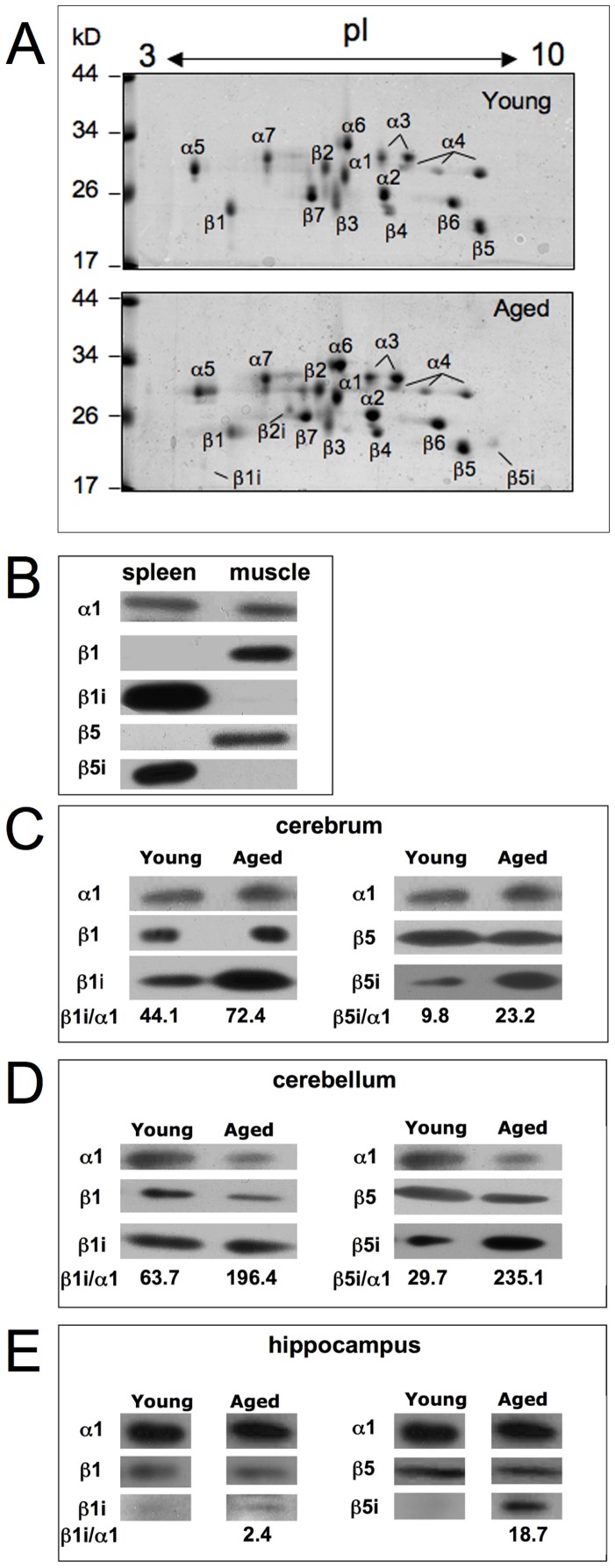
Figure 3. 2D-PAGE electrophoresis of 20S proteasome purified from cerebellum of young and aged rats.
Panel A. 30 µg of purified proteasome was applied to each gel, which were stained with Coomassie. Proteasome subunits were assigned according to our earlier investigations with proteasomes from rat liver [31]. The location of subunits β1i, β2i and β5i are indicated by a bar. Panel B–E. Standard- (β1 and β5) and immuno-subunits (β1i and β5i) in 26S proteasomes isolated from cerebrum (panel C), cerebellum (panel D), and hippocampus (panel E) from young and aged rats were detected by immunoblot analysis after SDS-PAGE. About 2–5 µg of 26S proteasome was subjected to the electrophoresis gels. The specificity of the antibodies was tested with 0.5 µg 20S proteasomes purified from rat spleen and muscle (panel B). As we are not aware of an antibody specific for rat proteasome β2 and β2i subunits, these proteins were not analysed here. As a loading control subunit α1 was identified in panel B–E and in panel C–E; the ratio of the signals (pixel intensity) of the immunosubunits β1i and β5i were calculated against α1 after their densitometric quantification by use of the ImageJ software.