Figure 3. Effect of buffer, temperature, and pH on 2D 1H-15N HSQC NMR spectra of a disulfide-rich venom peptide (Step 9).
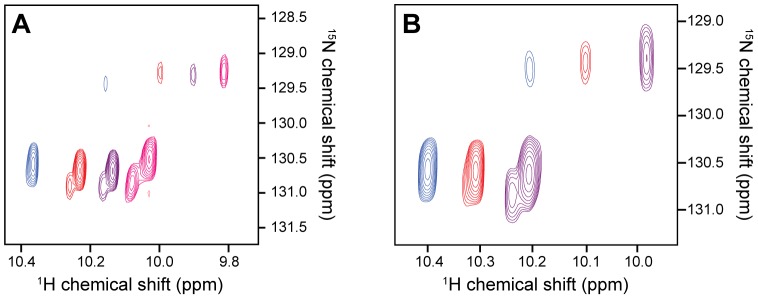
(A) Overlays of the downfield region of 2D 1H-15N HSQC spectra of a spider-venom peptide (46 residues, 4 disulfide bonds) [90] acquired at 25°C using different buffers and pH: 20 mM MES pH 6 (pink); sodium phosphate, pH 6 (purple); 20 mM sodium acetate, pH 5 (red); 20 mM sodium citrate, pH 4 (blue). This region of the spectrum shows the sidechain 1H-15N correlation for the single Trp residue in this peptide. (B) Effect of temperature on the same resonance. Spectra were acquired in 20 mM citrate, pH 4 at the following temperatures: 10°C (purple); 25°C (red); 40°C (blue). At low pH and high temperature the equilibrium is shifted towards a single conformer, compared to the three conformers apparent at lower temperature.
