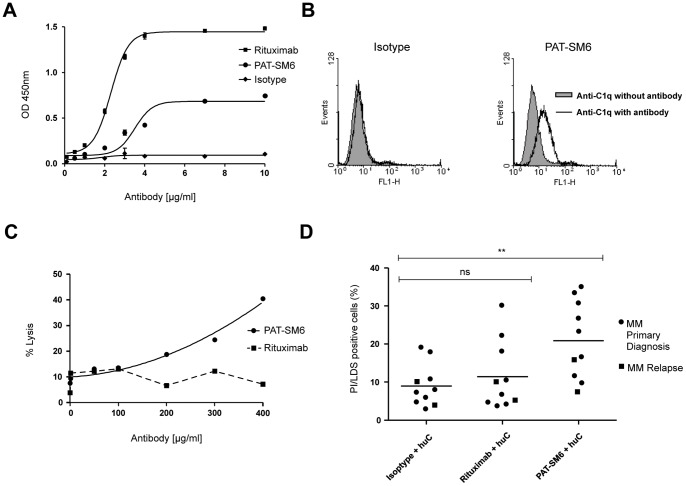
Figure 4. PAT-SM6 binds to C1q and mediates complement deposition and activation on MM cell lines and patients material.
A, Sandwich ELISA: For C1q binding analysis, plates were coated with PAT-SM6 or controls (isotype or Rituximab). After blocking, plates were incubated with human C1q followed by sheep anti human C1q-HRP. PAT-SM6 showed clear but moderate binding to C1q compared to the isotype control. Rituximab displayed a stronger C1q binding capacity. B, PAT-SM6 mediated deposition of C1q on the surface of OPM-2 cells assessed by using human C1q and detecting antibodies in FACS. C1q deposition was clearly improved with PAT-SM6 compared to cells without antibody treatment or isotype control. C, CDC activity was determined using the alamar blue assay. OPM-2 cells were incubated with human complement (huC), PAT-SM6 or controls for 2 hours followed by alamar blue solution and the amount of lysed cells was analysed with a fluorescence reader. A dose dependent killing kinetic was observed. D, CD138-purified primary MM cells at primary diagnosis (n = 8, dots) or relapse (n = 2, rectangle) were incubated with human complement, PAT-SM6 or controls (isotype, Rituximab) for 2 h and the amount of lysed was assessed by FACS (Propidium iodide/LDS75). In the PAT-SM6 treated samples a moderate killing was observed, whereas Rituximab showed no significant cytotoxicity.