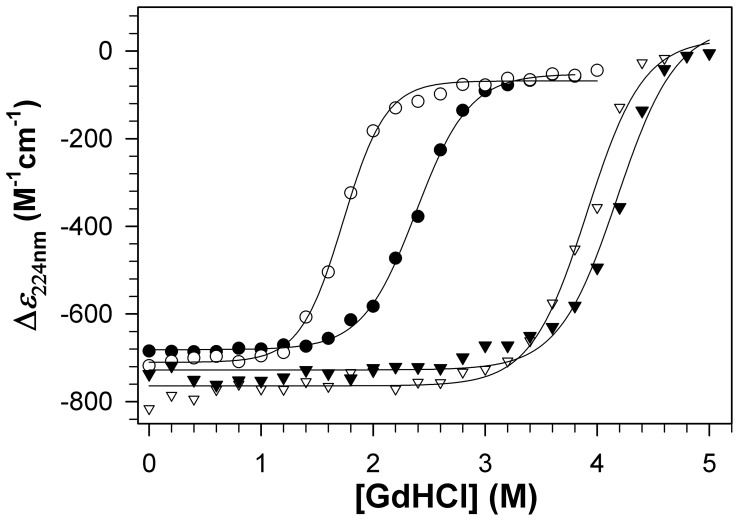
Figure 7. RP-mutant CaMnSODc is more subject to GdHCl-induced unfolding than the wild type.
The molar CD at 224 nm was used to monitor changes in α-helical structure content as a function of [GdHCl]. The enzymes were WT ScMnSOD (solid triangle), K182R, A183P ScMnSOD (hollow triangle), WT CaMnSODc (solid circle) and K184R, L185P CaMnSODc (hollow circle). The sample solutions contained 0.2 mg/mL (monomer concentration) MnSOD in 25 mM potassium phosphate (pH 7.4).