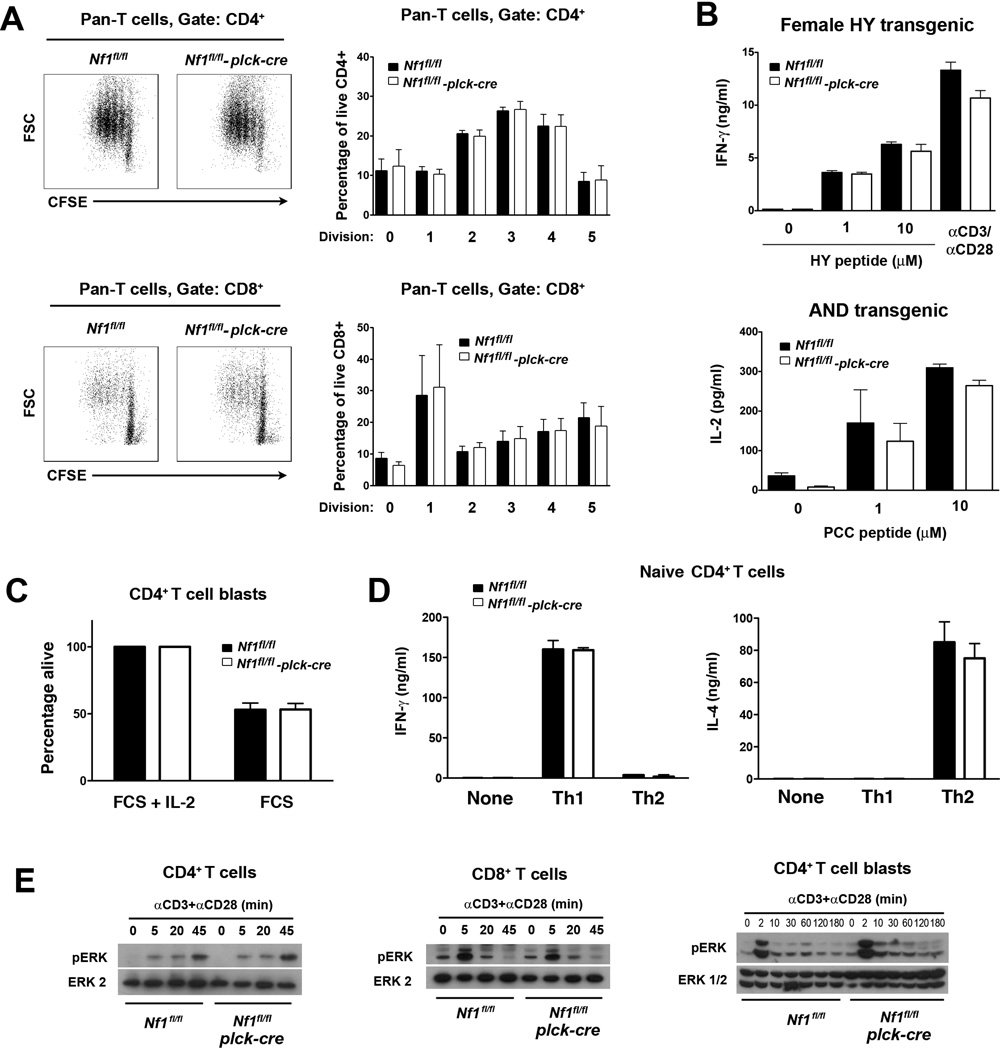
Fig. 7.
Function of NF1-deficient T cells. All experiments were performed with littermate Nf1flfl and Nf1fl/fl plck-cre non-TCR Tg, AND TCR Tg or HY TCR Tg mice. A, Purified pan T cells (CD4+ and CD8+) from non-TCR Tg mice were labeled with CFSE and stimulated with CD3 and CD28 mAb. Flow cytometry plots at left show representative CFSE fluoresence versus forward scatter upon gated CD4+ and CD8+ T cells after 72 h culture. Bar graphs at right show the mean percentage plus 1 SEM of CD4+ or CD8+ T cells at the indicated number of divisions at 72 h (n=4 mice of each genotype). B, Purified CD8+ HY TCR Tg T cells from spleens of female HY TCR Tg mice (all naïve CD44lo) and purified naïve CD44lo T cells from spleens of AND TCR Tg mice were stimulated with HY peptide-pulsed H-2b APC or PCC peptide-pulsed H-2k APC respectively. Concentrations of IFN-γ or IL-2 in culture supernatants were determined by ELISA after 48 h. Bar graphs show mean concentration of cytokines plus 1 SEM (n=3 mice of each genotype). C, CD4+ T cell blasts from non-TCR Tg mice were cultured in complete medium with or without IL-2 for 48 h at which point the percentage of viable cells was by determined by flow cytometry. Viability in the absence of IL-2 was normalized to viability in the presence of IL-2. Shown is mean percent viability + 1 SEM (n=3 mice of each genotype). D, Purified CD44− naïve CD4+ T cells from non-TCR Tg mice were stimulated for 5 d under Th1 or Th2 polarizing conditions. Cells were harvested and restimulated with CD3 mAb for 24 h. Concentrations of IFN-γ and IL-4 in culture supernatants were determined by ELISA. Shown are mean concentrations of cytokines plus 1 SEM for each polarizing condition (n=3 mice each genotype). E, CD4+, CD8+ T cells or CD4+ T cell blasts were stimulated with CD3 and CD28 mAb for the indicated times. Activation of the Ras-MAPK pathway was determined by Western blotting using a phospho-ERK antibody. Results are representative of three repeat experiments.