Abstract
Appetitive behavior is stronger when organisms are given a variety of foods than when they are repeatedly given the same food (the variety effect). Two experiments examined the variety effect in an operant food-seeking task. In both experiments, rats received a 45-mg food pellet for every 4th lever press over a series of daily 30-min sessions. The rats responded at a high rate early in the session, but the rate declined systematically over time within the session. In Experiment 1, alternating unpredictably between grain and sucrose pellets caused a higher level of responding, and a slower within-session decline in responding, than presenting either type of pellet consistently. In groups receiving one pellet consistently, a switch to the alternate pellet caused lawful changes in response rate that reflected both habituation and incentive contrast processes. In Experiment 2, an experimental group received grain only and sucrose only in daily alternating sessions. In sucrose sessions, they responded more than controls that always received either sucrose or grain (a type of variety effect); in grain sessions, they responded less than the controls. The results indicated a within-session variety effect that was controlled by habituation processes and a between-session variety effect that was controlled by incentive contrast. Both types of processes can come into play when organisms are exposed to food variety.
Keywords: Variety effect, food habituation, stimulus specificity, incentive contrast
Food consumption is higher when the meal or diet includes a variety of foods instead of a single food (e.g., Raynor & Epstein, 2001). This variety effect has been demonstrated in both humans and animals, and is one of many factors that might influence eating and overeating. One explanation of the variety effect is that exposure to a mixture of foods might retard the organism’s habituation to food. When organisms are repeatedly given the same food, consumption or instrumental responding for it decreases (e.g., Epstein, Temple, Roemmich, & Bouton, 2009). Exposure to different foods might slow the habituation process. For example, Temple, Giacomelli, Roemmich, and Epstein (2008) found that food variety influenced the rate of habituation in children engaged in a food-seeking task. Children received food rewards for playing a simple video game. When the food varied from occasion to occasion, the decline in response rate observed within a 30-min test session was slower than when the food was never varied. Similar results have been shown with adults (Myers Ernst & Epstein, 2002). In addition to demonstrating the influence of variety on food habituation, these studies, as well as others, suggest that habituation processes influence motivated food-seeking, that is, instrumental (operant) behavior that is reinforced by food.
Several well-known features of habituation could contribute to the variety effect. First, habituation is “stimulus-specific”; responding might recover from habituation to some extent whenever the food is changed (e.g., Epstein, Saad, Handley, Roemmich, Hawk, & McSweeney, 2003). Second, repeatedly changing the food might repeatedly cause dishabituation (e.g., Epstein, Rodefer, Wisniewski, & Caggiula, 1992), the recovery of responding that occurs to an habituated stimulus when another stimulus is presented. Third, presenting a variety of foods would increase the interval between successive presentations of the same food. Given stimulus-specificity, this might slow down the habituation of responding to a food, because the rate of habituation is generally thought to decrease by increasing the spacing between trials (e.g., Rankin et al., 2009). By any or all of these mechanisms, food variety might maintain appetite by slowing down the trial-to-trial habituation to food.
As in humans, food-seeking in animals may be influenced by food habituation. For example, many experiments have shown that food-reinforced operant behavior (e.g., lever pressing) can systematically decline within an experimental session (e.g., McSweeney, Hinson, & Cannon, 1996; McSweeney & Murphy, 2009; McSweeney & Swindell, 1999). McSweeney and colleagues have presented evidence to suggest that the decline can be due at least partly to habituation to the reinforcer. However, the variety effect has received relatively little attention in animal instrumental food habituation paradigms. McSweeney, Murphy, and Kowal (2004) found that varying the duration of access to a food reinforcer can slow down the habituation of operant responding in pigeons and rats. But we know of only one experiment that studied the effects of qualitative variation in the appetitive reinforcer on habituation in an instrumental task (Melville, Rue, Rybiski, & Weatherly, 1997, Experiment 1). In that experiment, rats were reinforced for lever pressing with a grape flavored liquid. Variety was manipulated by substituting one of three pellet reinforcers (each a random third of the time) for the liquid; different groups received pellets on different percentages of the occasions. Although “variety” manipulated this way slowed the rate of habituation, the result is equally consistent with the possibility that the food pellets were merely more reinforcing than the grape solution they replaced. There is surprisingly little unambiguous evidence of the variety effect in animal food-seeking habituation paradigms.
The present experiments therefore studied the effects of variety on habituation in an operant learning task in rats. They extended a procedure introduced by Aoyama and McSweeney (2001). Over a series of daily 30-min sessions, the rats received a 45-mg food pellet for every fourth lever-press response (a Fixed Ratio [FR] 4 reinforcement schedule). As reported by Aoyama and McSweeney, the rate of lever pressing at the start of each session was very rapid, but declined systematically over time within the session. Although several factors could contribute to that decline (e.g., fatigue and/or satiation), a role for habituation was suggested by the fact that several dishabituating events (e.g., withdrawing the lever from the chamber for 3 min) introduced in the middle of the session increased the rate of responding subsequently. We reasoned that if the within-session decline were due to food habituation, then it should also be slowed by providing a mixture of different food pellets—a variety effect. We also reasoned that substituting an alternative pellet for the habituated pellet in the middle of the session should lead to an increase in responding—a stimulus-specificity effect. Although stimulus-specificity has been shown with the habituation of food consumption in animals (e.g., Swithers & Martinson, 1998), we are not aware of studies that have demonstrated it in a food-seeking (operant) task.
Experiment 1
The first experiment examined variety and stimulus-specificity effects in the Aoyama and McSweeney preparation. Three groups of rats were allowed to lever press on an FR-4 schedule over a series of daily 30-min sessions. During each session, an experimental (variety) group received an unpredictable mixture of 45-mg sucrose and grain-based pellets for lever pressing. Two control groups received only grain and only sucrose pellets in alternating sessions. One group received grain pellets on odd-numbered days and sucrose pellets on even-numbered days, while the other received sucrose on odd-numbered days and grain on even-numbered days. On any day, we could thus compare the local rates of food-seeking in animals that were receiving an unpredictable mix of grain and sucrose with rats that were consistently receiving either grain or sucrose. Because the control groups had received the alternate pellet type on the previous day, cumulative exposure to the two reinforcers was approximately controlled. Part way through a final test session, one of the controls was switched from grain to sucrose, and the other from sucrose to grain. Note that the “new” pellet was approximately as familiar as the old. The Variety group was switched from intermixed grain and sucrose to consistent sucrose pellets only. The switch to sucrose, which was potentially a better reinforcer than grain, provided a conservative test of whether switching from variety to a consistent single pellet would decrease the rate of instrumental responding.
The habituation analysis makes at least three clear predictions in the present experiment: There should be (1.) a slower decline in response rate each session in the variety group than in the controls; (2.) an increase in responding in the final test when the controls were switched to the alternate pellet mid-session; and (3.) a decrease in response rate in the final test when the variety group was switched to consistent sucrose.
Method
Subjects
Thirty-two naive female Wistar rats purchased from Charles River Laboratories (St. Constance, Quebec) participated in the present study. They were between 75 and 90 days old at the start of the experiment and were individually housed in suspended wire mesh cages in a room maintained on a 16:8-h light:dark cycle. Throughout the experiment, the rats were food deprived to 80% of their initial body weights via small daily feedings of the maintenance chow, P500 Prolab RMH 3000 (PMI Nutrition International, Brentwood, MO).
Apparatus
The apparatus consisted of two unique sets of four conditioning chambers (Med Associates, St. Albans, VT, model ENV-008-VP). All boxes measured 30.5 × 24.1 × 23.5 cm (length × width × height). The floor was made of stainless steel grids (0.48-cm diameter) and the ceiling and sidewalls were made of clear acrylic plastic. The front and rear walls were made of brushed aluminum. A recessed 5.1 × 5.1 cm food cup was centered in the front brushed aluminum wall 2.5 cm above the floor. In both sets of boxes, a retractable lever (4.8 cm long and positioned 6.2 cm above the floor grid) was positioned to the right of the food cup. When extended, the lever protruded 1.9 cm from the front wall. A 28-V panel light (2.5 cm in diameter) was attached to the wall 10.8 cm above the floor and 6.4 cm to the left and right of the food cup. Ventilation fans provided background noise of 65 dB.
The two sets of conditioning chambers had unique features that allowed them to be used as different contexts (counterbalanced). In one set of boxes, one acrylic plastic sidewall had black diagonal stripes, 3.8 cm wide and 3.8 cm apart. The ceiling had similarly spaced stripes oriented in the same direction. The chambers were illuminated by one 7.5-W incandescent bulb mounted to the ceiling of the conditioning chamber, 34.9 cm from the grid floor, near the back wall. A distinct pine odor was continuously presented by placing 5 ml of Pine-Sol (Clorox Co., Oakland, CA) in a dish outside the chamber. The floor grids were spaced 1.6 cm apart (center to center) on the same plane. The other set of boxes had no distinct visual cues and the floor grids were staggered such that odd- and even- numbered grids were mounted in two separate places, one 0.5 cm above the other. The chambers were illuminated by one 7.5-W incandescent bulb mounted to the ceiling of the conditioning chamber, 34.9 cm from the grid floor, near the front wall. The continuous odor cue was provided by 5 ml of Rite Aid lemon cleaner (Rite Aid Corp., Harrisburg, PA).
There were two reinforcers. One was a 45-mg grain-based food pellet (MLab Rodent Tablet [5TUM], from Test Diet, Richmond, IN, USA), and the other was a 45-mg sucrose pellet (Sucrose Tablet [5TUT], from TestDiet, Richmond, IN, USA). The different pellets were delivered by separate feeders that delivered their pellets to the same food cup. Previous research in this laboratory indicates little generalization between the two types of pellets. The extent to which the pellets generalize with the maintenance chow (delivered only in the home cage) is unknown. However, according to manufacturer specifications, the chow is very different from both pellet types in macronutrient composition. For the chow, the percentage of calories from protein, fat, and carbohydrates (respectively) were 26%, 14%, and 60%; for the grain-based pellets, the percentages were 69.8%, 30.2%, and 0%; and for the sucrose pellets, the percentages were 0%, 0%, and 100%.
The apparatus was controlled by computer equipment located in an adjacent room.
Procedure
Magazine training
On Day 0, all rats received magazine training during 30-min sessions that were conducted in Context A and then Context B (the sessions were spaced approximately 3 hrs apart). In each session, approximately 60 pellets were delivered to the rat noncontingently every 30 s (on average). The type of pellets presented in each context was determined by group membership (described below). The levers were retracted during these sessions.
Response training
On Day 1, lever press training began. The subjects were divided into three groups. Group Grain (n = 10) received grain pellets in Context A and sucrose pellets in Context B. Group Sucrose (n = 10) received sucrose pellets in Context A and grain pellets in Context B. (We delivered the grain-based and sucrose pellets in different contexts in these control groups in an effort to help separate their effects.) Group Mix (n = 12) received a mixture of grain and sucrose pellets in both Contexts A and B. Sessions alternated daily between Contexts A and B. In each session, two minutes after the rat was placed in the box, the lever was inserted and the reinforcement schedule was in effect until the lever was retracted 30 mins later. Lever presses were reinforced on a fixed ratio four schedule (FR-4). Hand shaping was not necessary. For rats in the mixture group, delivery of the grain and sucrose pellets was random throughout each session with the restriction that there could be no more than four consecutive presentations of a particular pellet type. Response training continued for 17 consecutive days with exposure to each context alternating across days. The 17th session, conducted in Context A, was designed as a baseline session for comparison with performance in the Test session conducted in the same context on the next day.
Test
Stimulus specificity was tested on Day 18 in Context A. During the first 10 min of the session, each rat received a treatment identical to that received during the baseline session on Day 17. At the end of min 10, Group Sucrose began receiving grain pellets on FR-4 (instead of sucrose) and Group Grain began receiving sucrose (instead of grain). Group Mix was switched from the unpredictable grain and sucrose pellets to sucrose pellets only. Responding was monitored for the 20 min after the pellet switch and compared with performance during the preceding baseline session. Previous unpublished research with this method has revealed remarkable stability in lever press performance over days at this point in training.
Data analysis
We focused on performance in Context A, where one of the control groups consistently received sucrose pellets and the other consistently received grain pellets. (Results in Context B, where the controls received the opposite pellets, were consistent and entirely complementary.) All results were evaluated with analyses of variance (ANOVAs) using p < .05 as the rejection criterion.
Results
Acquisition phase
Within-session patterns of food-seeking
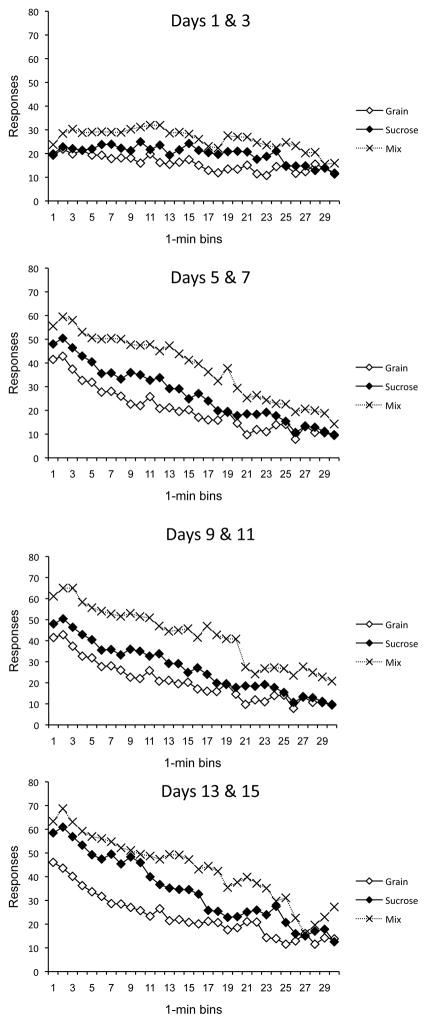
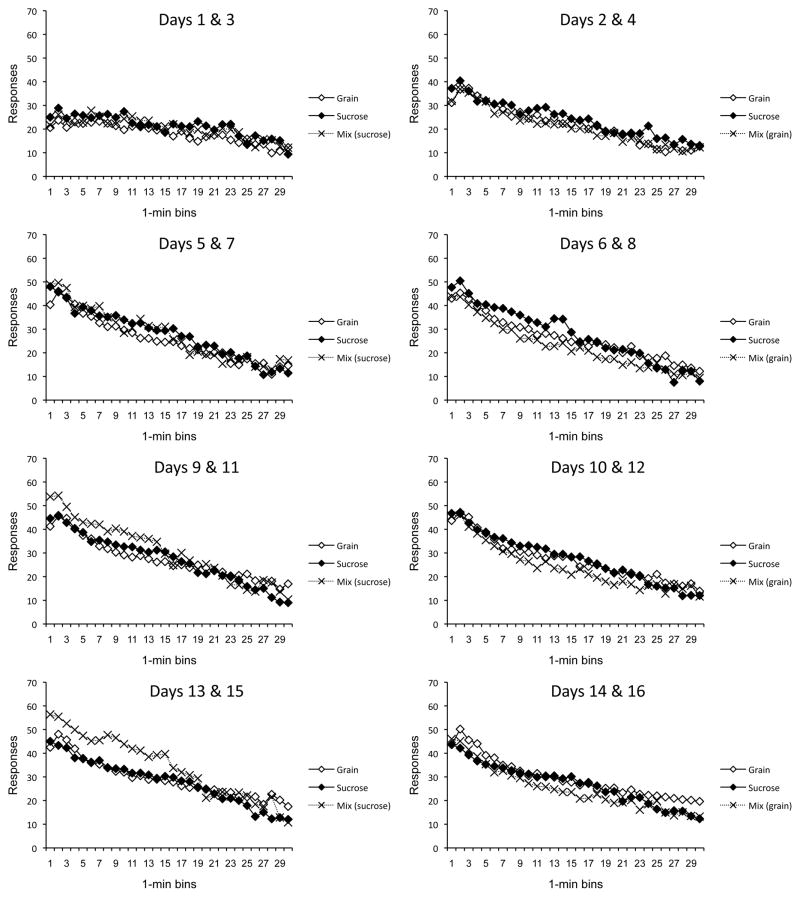
The results of the acquisition phase are presented in Figure 1, which shows responding for each group over time in the session (x axis) over successive two-day blocks in Context A (separate panels). The groups rapidly acquired the food-seeking response, and quickly revealed the pattern of decreasing responding within each session that was reported by Aoyama and McSweeney (2001). Although responding for sucrose pellets was eventually higher than that for grain pellets, responding for the mixture (Group Mix) was quickly and consistently higher than responding for either pellet alone. These impressions were confirmed by a 3 (Group) × 4 (2-Day Block) × 30 (Time Bin) ANOVA, which revealed main effects of Group, Day, and Time, minimum F(2, 29) = 60.16, MSE = 1575.41. The effect of Group interacted with both Day and Time, minimum F(58, 841) = 2.89, MSE = 112.59, and the effect of Day interacted with Time, F(87, 2523) = 13.06, MSE = 58.22. Finally, the three-way interaction between Group, Day, and Time also reached significance, F(174, 2523) = 1.20, MSE = 58.22. Planned pairwise comparisons revealed that Group Mix responded more than both Groups Grain and Sucrose in all panels of Figure 1 (all ps < .01). Group Sucrose was also higher than Group Grain in all the panels, ps < .05. Interestingly, like the differences between groups, the Time effect was also reliable as early as the first panel of Figure 1, i.e., Days 1 and 3, F (29, 841) = 8.20, MSE = 42.01.
Figure 1.
Mean lever press rates of the groups during each minute of the sessions conducted in Context A in Experiment 1. Each panel depicts a two-session (two-day) block. Group Grain = grain pellets in Context A; Group Sucrose = sucrose pellets in Context A; Group Mix = grain and sucrose pellets unpredictably in Context A.
The significant Group × Time and Group × Day × Time interactions suggest that the groups differed in the rate at which responding declined within the sessions. When we isolated the last 2-day block (bottom panel), when behavior was at asymptote, a Group × Time ANOVA confirmed a significant Group × Time interaction, F (58, 841) = 2.11, MSE = 88.86. Comparisons of Group Mix with each of the other groups during each bin (using a Bonferoni correction) indicated that Group Mix differed from Group Grain during bins 1–18 and from Group Sucrose only during bins 17 and 18. The latter result suggests that mixing grain pellets with sucrose pellets slowed down the response rate that otherwise occurred with sucrose alone.
A second ANOVA on the acquisition data compared responding for the grain and sucrose reinforcer over blocks in the two controls. A 2 (Group) × 4 (2-Day Block) × 30 (Time Bin) ANOVA revealed main effects of Group, Day, and Time, minimum F(1, 18) = 30.19, MSE = 1163.08. The effect of Group interacted with both Day and Time, minimum F(29, 522) = 3.87, MSE = 74.08, and the effect of Day interacted with Time, F(87, 1566) = 9.10, MSE = 48.24. Once again, the three-way interaction between Group, Day, and Time was significant, F(87, 1566) = 1.45, MSE = 48.24. The results suggest that sucrose pellets may be a more effective reinforcer than grain pellets, and that the difference increased over training. But most important, habituation occurring within each 30-min session was also clearly modulated by a variety effect.
Between-session patterns of food-seeking and energy consumption
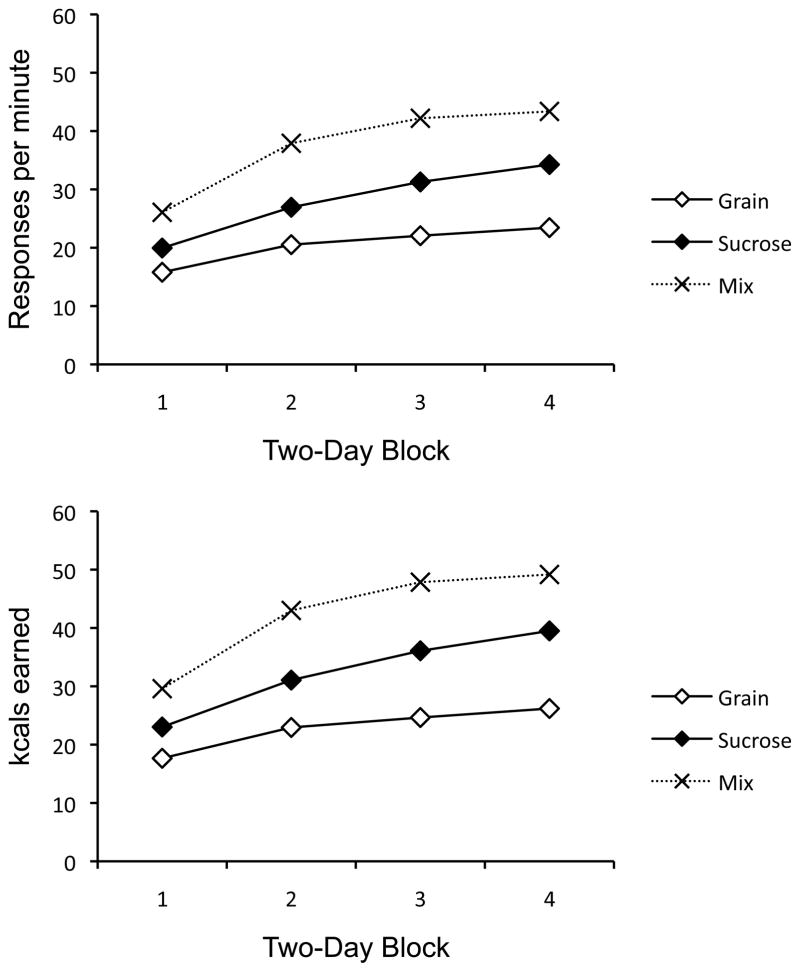
The lever-press rates of the groups during each 2-session block in Context A are replotted in the upper panel of Figure 2. This presentation of the data makes the between-session changes analyzed above especially clear. There was more responding for the mixture than for either pellet-type alone, and sucrose eventually controlled more responding than grain. But as is evident here, there was no tendency for food seeking to decline between sessions. That is, the results suggest no evidence of between-session habituation to the pellets.
Figure 2.
Mean lever press rates (top) and kcals earned (bottom) of the groups over two-day (two-session) blocks in Context A of Experiment 1.
The kcals earned by each group during the same session blocks are presented in the lower panel of Figure 2. (The values were calculated based on the manufacturer’s estimates of the kcal value of each pellet, which were 0.1485 for grain and 0.1534 for sucrose.) A 3 (Group) × 4 (2-Day Block) ANOVA revealed a significant main effect of Block, F(3,87) = 133.02, MSE = 10.30, and Group, F(2, 29) = 61.56, MSE = 27.95, as well as a significant interaction between the two, F(6, 87) = 8.04, MSE = 10.30. Separate ANOVAs on each day revealed a significant effect of Group on all days, minimum F(2, 29) = 16.08, MSE = 24.45. Pairwise comparisons revealed that Group Mix differed from both Groups Grain and Sucrose on all days (all ps < .01). Group Sucrose was also reliably higher than Group Grain on all blocks (all ps < .05).
Testing
Effects of the pellet switch in each group
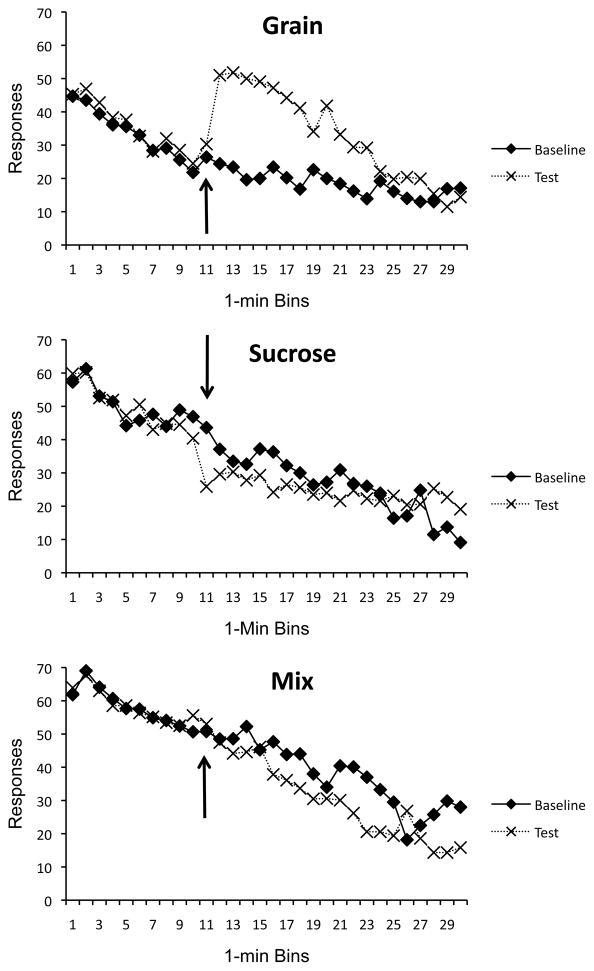
The effects of switching pellets part way through the test session on Day 18 are summarized in Figure 3, which compares responding that day with responding on the baseline day (Day 17) in each group in separate panels. During the first 10 min, each group received the same treatment on the baseline day and test day. An initial analysis confirmed that responding during this period was remarkably similar over the two days, and that group differences that had emerged in acquisition were retained. A 3 (Group) × 2 (Day) × 10 (Time Bin) ANOVA revealed a main effect of Group, F(2, 29) = 45.59, MSE = 725.36, Time, F(9, 261) = 56. 24, MSE = 42.14, and a Group × Time interaction, F(18, 261) = 2.30, MSE = 42.14, but no other main effects or interactions, including those involving Day, were significant, largest F(2, 29) = 1.42. Collapsing over the Day and Time Bin factors, pairwise comparisons (LSD) confirmed that Group Mix continued to respond more than both Group Grain and Group Sucrose, minimum t(29) = 3.33, and that Group Sucrose continued to respond more than Group Grain, t(29) = 5.59.
Figure 3.
Mean lever press rates during the baseline and session testing for stimulus specificity in Experiment 1. Each panel represents responding of one group. Grain = grain pellets during the baseline session and grain then sucrose pellets during the test; Sucrose = sucrose pellets during the baseline session and sucrose then grain pellets during the test; Mix = mixture of grain and sucrose pellets during the baseline session and mixture then sucrose pellets during the test. Arrows indicate the minute in which each group received the pellet switch during the test session.
More critically, responding during Mins 11–30 reflected the effects of changing the pellet reinforcer. As the figure suggests, the pellet switch affected responding in all three groups. In Group Mix, the switch from the unpredictable grain and sucrose to sucrose alone reduced response rate. This was confirmed by a 2 (Day) × 20 (Time Bin) ANOVA on data from this group, which revealed significant effects of Day, F(1, 19) = 9.16, MSE = 704.23, and of Time, F(19, 209) = 11.51, MSE = 232.30. The Day × Time interaction was not significant, F(19, 209) = 1.13. But clearly, switching from the mixture to what appears to be the preferred reinforcer (sucrose) caused a decrease in responding—a result that is consistent with the habituation perspective.
To test for a stimulus-specificity effect, the responding of Groups Grain and Sucrose were also studied during Mins 11–30. Instead of causing a uniform increase in responding, as suggested by habituation theory, the effect of the pellet switch depended on the group. A 2 (Group) × 2 (Day) × 20 (Time Bin) ANOVA revealed significant main effects of Day, F(1, 18) = 26.06, MSE = 260.70, and Time, F(19, 342) = 20.78, MSE = 89.30. The Time factor interacted with Day, F(19, 342) = 2.31, MSE = 96.47, as well as Group, F(19, 342) = 1.71, MSE = 89.30. The Day factor also interacted with the Group factor, F(1, 18) = 56.06, MSE = 260.70. And there was a reliable Group × Day × Time interaction, F(19, 342) = 7.47, MSE = 96.47. To understand the three-way interaction, separate 2 (Day) × 20 (Time) ANOVAs were conducted for each group. For Group Grain, there were main effects of Day, Time, and a significant Day × Time interaction, minimum F(19, 171) = 6.58, MSE = 98.80. The switch from grain to sucrose pellet thus increased responding in this group, and the difference between the test and baseline days was significant in time bins 12 – 18 and 20 – 23, minimum t(9) = 2.65. For Group Sucrose, the parallel ANOVA revealed a significant effect of Time, F(19, 171) = 8.25, MSE = 82.01, and a Day × Time interaction, F(19, 171) = 3.13, MSE = 94.17. The main effect of Day was not significant, F(1, 19) = 1.54. For this group, responding was significantly reduced on the Test Day in time bins 11, 15, 16, and 21, minimum t(9) = 3.52, but significantly higher in bins 28, 29 and 30, minimum t(9) = 2.38. The switch from sucrose to grain did increase response rate during the final minutes, but its more immediate effect was to depress food-seeking.
Comparison of responding in Mins 11–30 when the pellet was switched vs. not switched
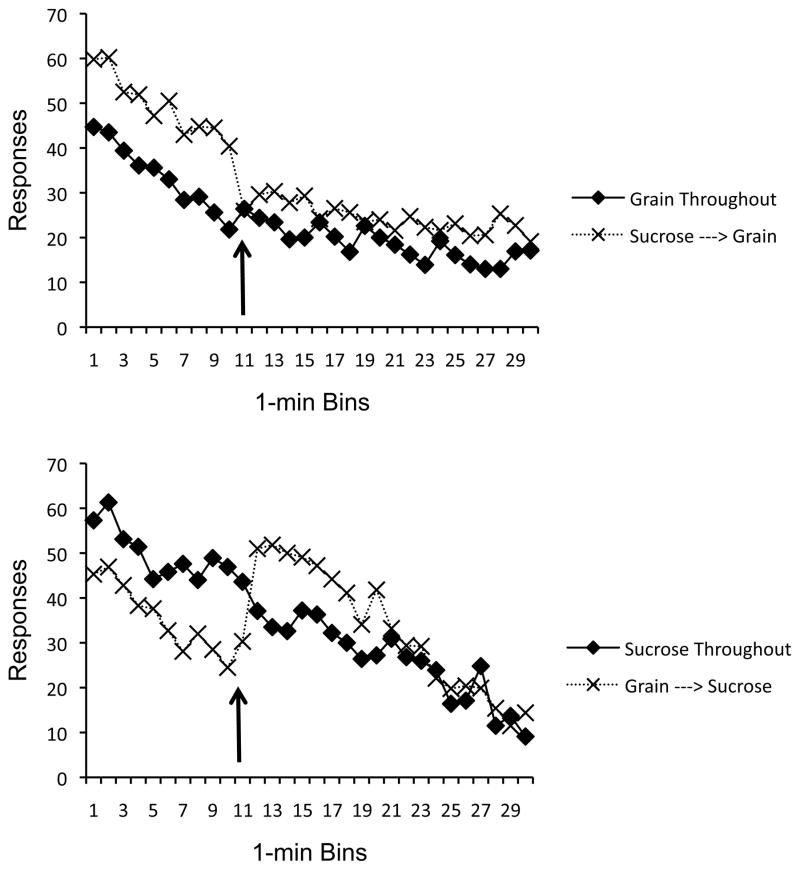
Interpretation of the stimulus-specificity effect was complicated by the fact that a switch from grain to sucrose enhanced responding but the switch from sucrose to grain largely depressed it. Such a pattern is consistent with well-known successive positive and negative incentive contrast effects (e.g., Flaherty, 1996; see Discussion). To further assess the role of stimulus-specificity, we also examined the results by comparing responding for each pellet in Mins 11–30 when it had been preceded by the same or a different reinforcer in the previous 10 mins (Mins 1–10). For instance, the upper panel of Figure 4 shows responding for grain pellets in Mins 11–30 when it had been preceded by 10 minutes of grain (Group Grain during the baseline session) or sucrose (Group Sucrose during the test session). Responding for grain in Mins 11–30 in the grain-grain condition, but not the sucrose-grain condition, was arguably under the influence of habituation from grain exposure in Mins 1–10. Isolating responding in Mins 11–30 for grain (upper panel), a 2 (Group) × 20 (Time) ANOVA revealed significant main effects of both Group, F(1, 18) = 17.56, MSE = 183.40, and Time, F(19, 342) = 4.45, MSE = 44.30. Thus, Group Sucrose responded more for its grain pellets at this time than did Group Grain, as predicted by habituation theory. The interaction was not significant. A parallel analysis confirmed the same pattern when we compared responding for sucrose in Mins 11–30 when it was preceded by 10 Mins of sucrose (baseline day in Group Sucrose) versus 10 Mins of grain (the test session for Group Grain, lower panel of Figure 4). A 2 (Group) × 20 (Time) ANOVA again revealed significant main effects of Time, F(19, 342) = 16.82, MSE = 141.52, Group, F(1, 18) = 5.27, MSE = 678.44, and a significant Group × Time interaction, F(1, 342) = 2.26, MSE = 141.52. Thus, a stimulus-specificity effect did emerge. Overall, the results thus tentatively suggest a role for both successive incentive contrast and habituation in creating the pellet switch results.
Figure 4.
Experiment 1’s stimulus specificity effect analyzed a different way. Top: A comparison of responding for grain throughout the session and grain after 10 mins of responding for sucrose. Bottom: A comparison of responding for sucrose throughout the session and sucrose after 10 mins of responding for grain. Arrows indicate the minute in which the groups received the pellet switch. See text for further explanation.
Discussion
The experiment produced several notable results. Consistent with Aoyama and McSweeney (2001), the rats began each session with a high rate of lever pressing which then declined systematically over the remainder of the session. The within-session decrease in response rate was evident early in training, e.g., on the first two-day block in Context A (Figure 1).
Several other results point to the role of within-session habituation to the pellet reinforcer. First, throughout acquisition there was a clear variety effect in which mixed presentation of grain and sucrose pellets produced more lever pressing than did either pellet alone. Like the decreasing response-rate effect, the variety effect was evident from the first two-day block of training (Figure 1). Although variety might generally increase the reinforcing value of the food pellets, one effect suggested by the present data was that it also slowed the within-session decline in response rate. Second, there was evidence of stimulus-specificity. That is, responding was higher for a pellet in Minutes 11–30 of the test if the rat had been switched to that reinforcer in Minute 10. It is worth noting that during stimulus-specificity testing the switched-to pellet was presented for the first time in the context in which testing occurred. It is therefore possible that its reinforcing value was enhanced by the fact that it was not expected in that context (cf. Wagner & Brandon, 1989). However, there was no complementary evidence that the pellets became less reinforcing as the rats were exposed to them during repeated sessions in the same context (e.g., Figure 2). That is, there was no evidence of a long-term, between-sessions habituation. A third result that was consistent with the habituation perspective was that rats switched to consistent sucrose pellets after exposure to the unpredictable grain/sucrose mixture slowed their response rate. Notably, that result occurred even though the sucrose pellet appeared to be especially reinforcing. The fact that a switch from variety to a single food can slow down pellet consumption may further support the idea that reducing variety in the diet can reduce overeating in humans (e.g., Epstein, Fletcher, O’Neill, Roemmich, Raynor, & Bouton, 2013).
The results also suggest that other motivational processes (besides habituation) influenced food-seeking. During acquisition, responding for sucrose pellets increasingly exceeded that for grain pellets. Although sucrose pellets might be more palatable than grain pellets, we have seen no evidence that the present sucrose pellets are unconditionally better reinforcers than the grain pellets (e.g., see Experiment 2, below; see also Winterbauer, Lucke, & Bouton, 2013, Experiment 3). Instead, as documented above, the sucrose superiority effect increased as a function of training. It is therefore possible that sucrose responding was enhanced by exposure to grain pellets on the alternate sessions because of a positive contrast effect—when organisms are exposed to a palatable food or flavor after exposure to positive but less palatable food or flavor, they may respond especially strongly to it (e.g., Flaherty, 1996). Interestingly, successive contrast effects have been demonstrated, as here, when the two incentives are presented in separate contexts and days (Flaherty, Hrabinski, & Grigson, 1990). A second type of contrast effect—negative contrast-- emerged when rats were briefly exposed to sucrose pellets and then switched to grain during the test session. This raises the possibility that the greater responding for sucrose than grain in Context A could have reflected both positive and negative contrast effects. That is, the rats might have responded more to sucrose because of recent exposure to grain and less to grain because of recent exposure to sucrose.
Definitive evidence of between-session incentive contrast effects would require that we compare the performance of a group alternated between grain and sucrose with groups that received the same reinforcer across sessions. The possibility was worth investigating in its own right, but it is also a possible mechanism that could contribute to the variety effect. First, notice that higher responding in sucrose sessions intermixed with grain sessions would itself be a type of variety effect. And second, if positive contrast for sucrose were stronger than negative contrast for grain, then a mixture of the two pellet types could generate a higher average level of responding to sucrose and grain, the form taken by the variety effect demonstrated in Experiment 1. Experiment 2 was therefore designed to examine possible between-session positive and negative contrast in more detail.
Experiment 2
Experiment 2 compared responding in rats given alternating sessions with grain and sucrose pellets with groups that received grain- or sucrose-only every day. If mixing the pellet types between sessions creates a positive contrast effect, then the alternating group should respond more for sucrose pellets than the group that received sucrose daily. If it creates a negative contrast effect, then the alternating group should respond less for the grain pellets than the group that received them daily. By averaging performance over pairs of sessions, it was also possible to ask whether variety between sessions led to slower daily habituation than presentation of the same food pellet daily.
Method
Subjects
The subjects were thirty-two naïve female Wistar rats purchased from the same vendor as those in the previous experiments. They were maintained under the same conditions.
Apparatus
The apparatus was the same as that in Experiment 1.
Procedure
Daily 30-min sessions, alternating between Contexts A and B, were run in a manner identical to those in Experiment 1. Group Grain (n = 10) received grain pellets in both Contexts A and B. Group Sucrose (n = 10) received sucrose pellets in both contexts. Group Mix (n = 12) received grain pellets in Context A and sucrose pellets in Context B, i.e., the treatment received by the control groups in Experiment 1. The experiment continued for 16 sessions.
Results
Within-session patterns of food-seeking
The results are presented in Figure 5, which shows responding of the groups over two-session blocks of training in each context. The left and right columns correspond to sessions in Contexts A and B, where Group Mix received sucrose and grain pellets, respectively. The data were analyzed with a Group (Grain vs. Sucrose vs. Mix) × Context (A or B) × Time Bin (30) × Session Block (4) ANOVA. There was no main effect of Group, F < 1, however, all other main effects and interactions were significant. Specifically, there were main effects of Context, Block, and Time, minimum F(1, 29) = 6.15, MSE = 354.94. The Context factor interacted separately with Group, Block and Time, minimum F(29, 841) = 2.96, MSE = 27.85. There was a difference between Group Mix’s performance in the two contexts. The Session Block factor also interacted with Group, and Time, minimum F(6, 87) = 5.75, MSE = 169.87. The Time factor also interacted with Group, F(58, 841) = 2.27, MSE = 71.34. All of the three way interactions between the factors were significant, minimum F(174, 2523) = 1.45, MSE = 31.75. There was also a significant 4-way interaction between Group, Context, Trial Block, and Time, F(174, 2523) = 1.20, MSE = 25.42. The four-way interaction is consistent with the impression suggested by the figure that Group Mix showed a differential pattern of responding in the two contexts that was primarily observed in early minutes of the later sessions.
Figure 5.
Mean lever press rates of the groups during each minute of the sessions conducted in Context A (left column) and Context B (right column) in Experiment 2. Each panel depicts a two-session (two-day) block. Group Mix alternated between sucrose pellets in Context A and grain pellets in Context B. Groups Grain and Sucrose consistently worked for grain and sucrose pellets, respectively, every day.
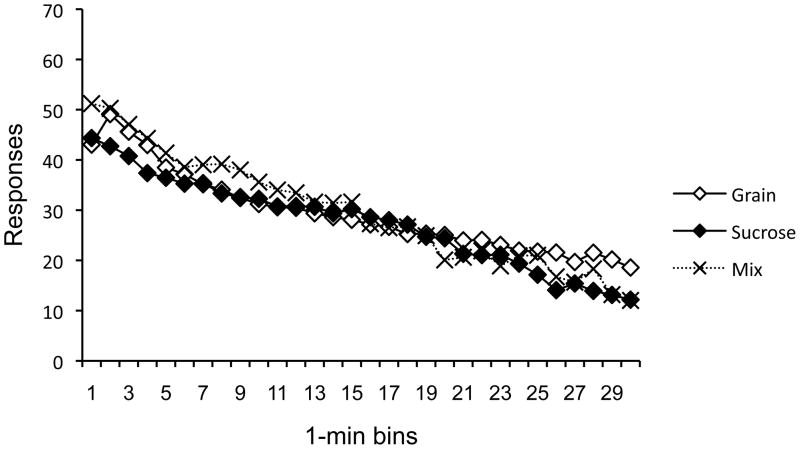
Analysis of an averaged between-session variety effect
Did Group Mix’s pattern of responding in the sucrose- and grain-pellet contexts average to produce an overall between-session variety effect? To address the question, we averaged the asymptotic performance in the final two sessions in each context (the bottom two panels of Figure 5), where the contrast effects were strongest. The averaged data are presented in Figure 6. First consider the data from the final two-session blocks in Contexts A and B, respectively. In Context A, where Group Mix worked for sucrose, a 3 × 30 ANOVA revealed a main effect of Time, F(29, 841) = 83.14, MSE = 39.03, Group, F(2, 29) = 6.46, MSE = 590.36, and a Group × Time interaction, F(58, 841) = 3.56, MSE = 39.03. Pairwise comparisons revealed that Group Mix was reliably higher than both Group Sucrose and Grain, minimum t(29) = 2.72, confirming positive contrast. Group Grain and Group Sucrose were not reliably different, p = .52. In Context B, where Group Mix worked for grain, a 3 × 30 ANOVA revealed a main effect of Time, F(29, 841) = 103.28, MSE = 22.84, Group, F(2, 29) = 5.37, MSE = 315.14, and a Group × Time interaction, F(58, 841) = 1.68, MSE = 22.84. Pairwise comparisons revealed that Group Mix was reliably lower than Group Grain, t(29) = 3.27, suggesting negative contrast for grain. Group Mix did not differ from Group Sucrose and Group Sucrose did not differ from Group Grain, largest t(29) = 1.81. Turning to the averaged data (Figure 6), there was no indication that Group Mix responded more overall than the other groups. A 3 × 30 ANOVA revealed a main effect of Time, F(29, 841) = 131.83, MSE = 20.96, and a Group × Time interaction, F(58, 841) = 2.81, MSE = 20.96. Inspection of Figure 6 suggests little consistent pattern. Moreover, the main effect of Group did not approach significance, p = .27, and pairwise comparisons did not reveal any overall group differences, largest t(29) = 1.55, p = .13.
Figure 6.
Mean lever press rates of the groups during Days 13–16 in Experiment 2. Group Mix’s response rates reflects responding for sucrose on Days 13 and 15 and grain on Days 14 and 16. Groups Grain and Sucrose consistently worked for grain and sucrose pellets, respectively, every day.
Between-session patterns of food-seeking and energy consumption
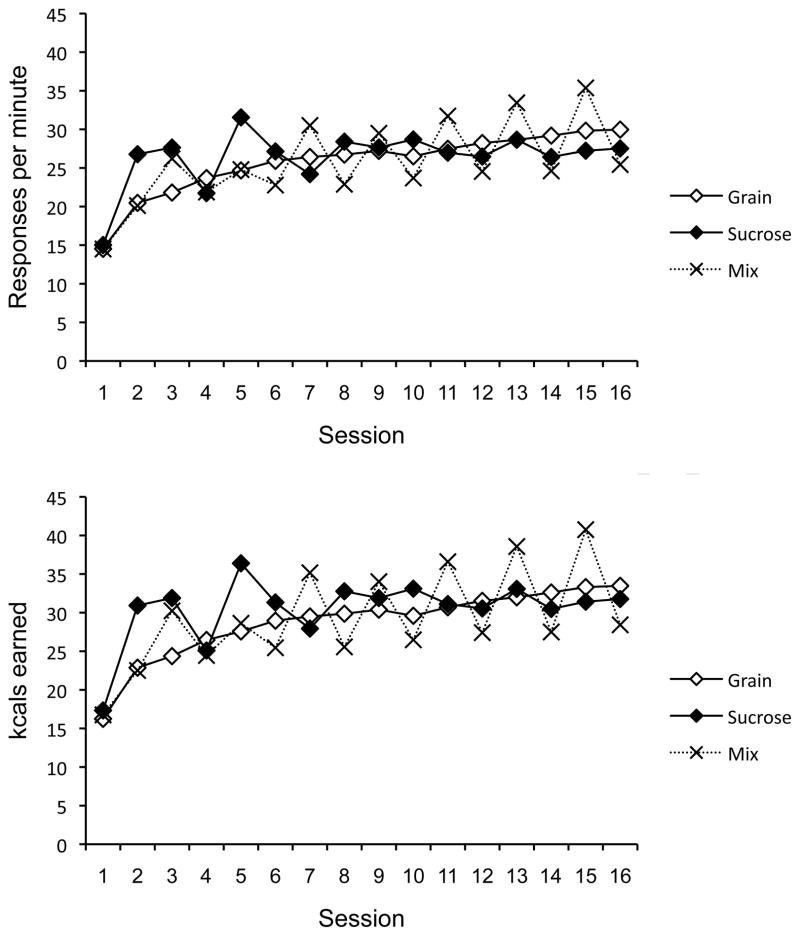
The lever-press rates of the groups during each daily session are presented in the upper panel of Figure 7. Recall that Group Mix received sucrose pellets in the odd-numbered sessions and grain pellets in the even-numbered sessions. This presentation of the data makes it easier to see that Group Mix’s differential responding for sucrose and grain in the two contexts grew as a consequence of training.
Figure 7.
Mean lever press rates (top) and kcals earned (bottom) of the groups over daily sessions in Experiment 2. Group Mix earned sucrose pellets on odd-numbered days and grain pellets on even-numbered days. Groups Grain and Sucrose consistently worked for grain and sucrose pellets, respectively, every day.
The kcals earned by each group during each session are presented in the lower panel of Figure 7. A 3 (Group) × 16 (Session) ANOVA revealed a main effect of Session, F(15, 435) = 50.71, MSE = 11.66, and a Session × Group interaction, F(30, 435) = 10.35, MSE = 11.66. The main effect of Group was not significant, F < 1. The Group × Session interaction was likely caused by the saw-tooth pattern of responding that had been evident in Group Mix. The sawtooth pattern was reliable. For example, on Day 15, the last day of sucrose pellets for Group Mix, Group Mix earned more kcals than either of the other groups, ps < .01, while Groups Grain and Sucrose did not differ (p = .413). On Day 16, the last day of grain pellets for Group Mix, Group Grain and Sucrose again did not differ (p = .328), but Group Mix was now significantly lower than Groups Grain, p < .01, and Sucrose (p = .051).
Discussion
As in Experiment 1, a reliable decreasing pattern of responding over minutes in each session rapidly emerged (Aoyama & McSweeney, 2001). Responding for either sucrose or grain pellets throughout training produced a stable pattern of responding within and between sessions. However, alternating between sucrose and grain pellets over sessions (Group Mix) led to more responding for sucrose and less responding for grain. These effects are consistent with between-session positive and negative successive contrast effects, respectively. It is worth noting again that the enhanced responding for sucrose in Group Mix can be viewed as a between-session variety effect—because it clearly depended on exposure to the grain pellets on the intervening sessions. However, Group Mix’s average responding for sucrose and grain pellets did not exceed that of the other groups. This result provides little support for the idea that between-session mixing of the present sucrose and grain pellets yielded a variety effect that was directly analogous to the one observed when the pellet types were mixed (and responding for them was averaged) within sessions (Experiment 1). Thus, although the present experiment uncovered a between-session variety effect that was arguably due to positive contrast, it provided no support for the possibility that an averaged effect, like the one documented in Experiment 1, may follow from the net combination of positive and negative contrast. The within-session effect observed in Experiment 1 may be more attributable to the effect of variety on within-session habituation to food.
General Discussion
In the present experiments, the rate of lever pressing for grain or sucrose pellets declined rapidly within daily 30-min sessions, as reported by Aoyama and McSweeney (2001). However, there was evidence that the rate decline occurred more slowly when the rats received pellets that varied unpredictably between grain and sucrose (Experiment 1). This new demonstration of the variety effect suggests that the within-session decrease in lever pressing rate that otherwise occurred was not merely a result of simple fatigue or food satiation. Instead, it is consistent with the idea that there was within-session habituation to the food reinforcers (e.g., McSweeney et al., 1996; McSweeney & Murphy, 2009; McSweeney & Swindell, 1999). Interestingly, there was no evidence that habituation occurred between sessions with the current method. However, the higher rate of within-session food-seeking in Group Mix could have long-term consequences, because it was demonstrably linked to higher energy intake. Thus, the type of within-session variety effect found here can in principle have an important effect on calorie consumption and (possibly) weight gain. In this regard, it may be significant to note that when we switched from the grain/sucrose mixture to sucrose pellets only, the rats decreased their work rate and consumption of food. As noted earlier, such a result is consistent with the possibility that exposure to single foods may in principle decrease overeating that may be supported by food variety (e.g., Epstein et al., 2013).
Experiment 1 also produced evidence of a within-session stimulus-specificity effect. Specifically, when rats worked for the same type of pellet throughout the session, they pressed at a lower rate during minutes 11–30 than did rats that were switched to the same pellet after 10 mins of earning the other type. This result is further consistent with the idea that the within-session decrease in food-seeking rate was a result of habituation to the pellet reinforcer. And it is worth noting that, as observed previously, stimulus-specificity could play a role in producing the variety effect.
It should be noted, however, that the detection of stimulus-specificity was made difficult by the fact that a switch to grain pellets after 10 mins of working for sucrose first depressed, rather than enhanced, the animal’s rate of food seeking. Although the switch from sucrose to grain ultimately increased responding in the last few minutes of the test session, the effects of the pellet switch highlighted the presence of incentive contrast effects (e.g., Flaherty, 1996). Our appreciation of incentive contrast was extended by Experiment 2, which confirmed the occurrence of both positive and negative contrast between sessions. Specifically, a group that responded for sucrose and grain in alternating sessions worked more for sucrose than a control that always received sucrose (positive contrast) and less for grain than a control that always received grain (negative contrast). Thus, when animals are exposed to food variety between sessions, contrast can clearly play a role. And it is interesting to note that the higher responding for sucrose in the group given alternating grain and sucrose sessions can itself be viewed as a variety effect. Although there was no evidence of between-session habituation with the current methods, exposure to variety (the grain sessions intermixed with sucrose sessions) caused the rats to consume more sucrose than the sucrose group that received no variety.
The presence of incentive contrast serves to remind us that food variety can engage motivational processes that go beyond those that influence habituation. Is it possible that incentive contrast, rather than slowed habituation per se, can account for the increase in food consumption that variety caused here? In at least one case, the answer is “yes”; the variety effect represented in higher responding for sucrose on sucrose sessions intermixed with grain sessions (Experiment 2) is clearly linked to positive contrast. But it seems less likely that contrast can explain the within-session variety effect observed in Experiment 1. For one thing, there was no evidence of a variety effect when we averaged over separate sessions of sucrose and grain responding in Experiment 2; in that case, the positive influence of grain on sucrose responding did not outweigh the negative influence of sucrose on grain responding. And other aspects of the within-session data seem especially consistent with the habituation explanation. Consistent with the habituation perspective, variety affected the rate at which habituation (or at least the decline in responding) occurred over time within the session, although it is possible that this interaction between pellet type and time was forced by a ceiling effect on response rate for the mix early in the session. Perhaps more important, the fact that there was more responding in rats that were switched from sucrose to grain mid-session (compared with rats that responded only for grain) suggests that habituation processes might play a role even in the presence of a negative contrast effect.
The results add to the extensive literature on habituation to foods in animals and humans (Epstein et al., 2009). The fact that switching from the grain/sucrose pellet mixture to sucrose alone reduced response rate (Experiment 1) may have especially important implications for human eating. Sucrose is known to be an important determinant of food reinforcement in humans (Avena, Rada, & Hoebel, 2008; Epstein, Carr, Lin, & Fletcher, 2011), and the results suggest that responding for even a powerful food reinforcer can be weaker when it is presented alone than in the context of variety. Perhaps similarly, many human studies that have shown higher responding for variety than for a single food have made sure that the single food is the most palatable of the foods available to the subject (Epstein et al., 2009; Temple et al., 2008). The findings thus encourage optimism about the clinical effectiveness of reducing food variety, since there should be minimal resistance to an intervention that reduces access to a variety of foods so that an overweight person can consume their favorite food.
In summary, the present experiments uncovered evidence of both within-session (Experiment 1) and between-session (Experiment 2) variety effects. The within-session variety effect may be best interpreted as a slowing-down of the short-term habituation to food. The between-session effect, on the other hand, is best interpreted as a positive contrast effect in which pressing for grain on alternate sessions motivated the rat to work more for sucrose. Both types of mechanisms may come into play when organisms are exposed to food variety.
Rats habituated to grain and/or sucrose pellets as they worked for them daily in an operant task
A mix of the two pellets motivated more responding than did either pellet alone
Manipulated within sessions, food variety slowed down the short-term habituation to food
Manipulated between sessions, variety created incentive contrast effects
Acknowledgments
This research was supported by Grants 9RO1 DA033123 from the National Institute on Drug Abuse to MEB and 1U01 DK088380 from the National Institute of Diabetes and Digestive and Kidney Diseases to LEH. The participation of Samuel León (who visited the University of Vermont from the University of Jaén) was enabled by projects SEJ2007-267053/PSIC and BES-2008-003634 from the Spanish Ministry of Science and Innovation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Mark E. Bouton, University of Vermont
Travis P. Todd, University of Vermont
Olivia W. Miles, University of Vermont
Samuel P. León, University of Vermont
Leonard H. Epstein, University at Buffalo
References
- Aoyama K, McSweeney FK. Habituation may contribute to within-session decreases in responding under high rate schedules of reinforcement. Animal Learning & Behavior. 2001;29:79–91. [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: Behavioral and neurochemical effects of intermittent, excessive sugar intake. Neuroscience and Biobehavioral Reviews. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Carr KA, Lin H, Fletcher KD. Food reinforcement, energy intake, and macronutrient choice. American Journal of Clinical Nutrition. 2011;94:12–18. doi: 10.3945/ajcn.110.010314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Rodefer JS, Wisniewski L, Caggiula AR. Habituation and dishabituation of human salivary response. Physiology and Behavior. 1992;51:945–950. doi: 10.1016/0031-9384(92)90075-d. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Saad FG, Handley EA, Roemmich JN, Hawk LW, McSweeney FK. Habituation of salivation and motivated responding for food in children. Appetite. 2003;41:283–289. doi: 10.1016/s0195-6663(03)00106-5. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Temple JL, Roemmich JN, Bouton ME. Habituation as a determinant of human food intake. Psychological Review. 2009;116:384–407. doi: 10.1037/a0015074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Fletcher KD, O’Neill J, Roemmich JN, Raynor H, Bouton ME. Food characteristics, long-term habituation and energy intake: Laboratory and field studies. Appetite. 2013;60:40–50. doi: 10.1016/j.appet.2012.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty CF. Incentive relativity. New York: Cambridge University Press; 1996. [Google Scholar]
- Flaherty CF, Hrabinski K, Grigson PS. Effect of taste context and ambient context changes on successive negative contrast. Animal Learning & Behavior. 1990;18:271–276. [Google Scholar]
- McSweeney FK, Hinson JM, Cannon CB. Sensitization-habituation may occur during operant conditioning. Psychological Bulletin. 1996;120:256–271. [Google Scholar]
- McSweeney FK, Murphy ES. Sensitization and habituation regulate reinforcer effectiveness. Neurobiology of Learning and Memory. 2009;92:189–198. doi: 10.1016/j.nlm.2008.07.002. [DOI] [PubMed] [Google Scholar]
- McSweeney FK, Murphy ES, Kowal BP. Varying reinforcer duration produces behavioral interactions during multiple schedules. Behavioural Processes. 2004;66:83–100. doi: 10.1016/j.beproc.2004.01.004. [DOI] [PubMed] [Google Scholar]
- McSweeney FK, Swindell S. General-process theories of motivation revisited: The role of habituation. Psychological Bulletin. 1999;125:437–457. [Google Scholar]
- Melville CL, Rue HC, Rybiski LR, Weatherly JN. Altering reinforcer variety or intensity changes the within-session decrease in responding. Learning and Motivation. 1997;28:609–621. [Google Scholar]
- Myers Ernst M, Epstein LH. Habituation of responding for food in humans. Appetite. 2002;38:224–234. doi: 10.1006/appe.2001.0484. [DOI] [PubMed] [Google Scholar]
- Rankin CH, Abrams T, Barry RJ, Bhatnagar S, Clayton DF, Colombo J, Coppola G, Geyer MA, Glanzman DL, Marsland S, McSweeney FK, Wilson DA, Wu C, Thompson RF. Habituation revisited: An updated and revised description of the behavioral characteristics of habituation. Neurobiology of Learning and Memory. 2009;92:135–138. doi: 10.1016/j.nlm.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynor HA, Epstein LH. Dietary variety, energy regulation, and obesity. Psychological Bulletin. 2001;127:325–341. doi: 10.1037/0033-2909.127.3.325. [DOI] [PubMed] [Google Scholar]
- Swithers SE, Martinson FA. Habituation of oral responding in adult rats. Behavioral Neuroscience. 1998;112:213–224. doi: 10.1037//0735-7044.112.1.213. [DOI] [PubMed] [Google Scholar]
- Temple JL, Giacomelli AM, Roemmich JN, Epstein LH. Habituation and within session changes in motivated responding for food in children. Appetite. 2008;50:390–396. doi: 10.1016/j.appet.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AR, Brandon SE. Evolution of a structured connectionist model of Pavlovian conditioning (AESOP) In: Klein SB, Mowrer RR, editors. Contemporary learning theories: Pavlovian conditioning and the status of traditional learning theory. Hillsdale, NJ: Lawrence Erlbuam Associates, Inc; 1989. pp. 149–189. [Google Scholar]
- Winterbauer NE, Lucke S, Bouton ME. Some factors modulating the strength of resurgence after extinction of an instrumental behavior. Learning and Motivation. 2013;44:60–71. doi: 10.1016/j.lmot.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]