Abstract
Objective
The purpose of this study was to determine the effects of electroacupuncture (EA) ST36 on food intake and body weight in Obese Prone (OP) rats compared to obese resistant (OR) strain on a high fat diet. The influences of EA on mRNA levels of pro-opiomelanocortin (POMC), transient receptor potential vanilloid type-1 (TRPV1), and neuronal nitric oxide synthase (nNOS) were also examined in the medulla regions and ST36 skin tissue.
Methods
Advanced EA ST36 was conducted in two sessions of 20 min separated by an 80 min interval for 7 days. Food intake and body weight were recorded in conscious rats every day. Real time PCR was conducted in the micropunches of the medulla regions and skin tissues at the end of the treatment.
Results
Food intake and body weight were significantly reduced by advanced EA ST36 in OP rats, but slightly decreased in OR strain and sham-EA rats. Advanced EA ST36 produced a marked increase in POMC mRNA level in the nucleus tractus solitarius (NTS) and hypoglossal nucleus (HN) regions. TRPV1 and nNOS mRNAs were simultaneously increased in the NTS/gracile nucleus regions and in the ST36 skin regions by the EA treatment in OP rats.
Conclusions
We conclude that advanced EA ST36 produces an up-regulation of anorexigenic factor POMC production in the NTS/HN, which inhibits food intake and reduces body weight. EA-induced expression of TRPV1-nNOS in the ST36 and the NTS/gracile nucleus is involved in the signal transduction of EA stimuli via somatosensory afferents-medulla pathways.
Keywords: Appetite regulation, POMC, TRPV, medulla, acupuncture
1. Introduction
The prevalence of overweight and obesity in the United States and the world has grown dramatically in recent years [28,25,30]. Hyperphagia is a major ingestive disorder developed in obese humans [14,25,30,], and in Obese Prone (OP) rats, a diet-induced obese model [11,20,39]. It has been shown that acupuncture and electroacupuncture (EA) have some effects in the reduction of body weight-obesity [3,17,43,44]. Recent studies have reported that EA stimulation of Zusanli (ST36) and Sanyinjiao (SP6) produced up-regulation of amphetamine-regulated transcript peptide expression in the arcuate nucleus associated with an inhibition of food intake and a reduction of body weight in Sprague-Dawley rats fed to obesity on a high-fat diet [37].
The nucleus tractus solitarius (NTS) is the principle sensory nucleus in the medulla and plays an important role in sensory and feeding regulation [15,19,38]. Recent studies have demonstrated that sensory stimulation of the hindlimb somatic afferent modifies neuronal activities in the NTS [38]. The gracile nucleus (GN) receives peripheral somatosensory nociceptive afferents projecting from the hindlimb [18,40], and stimulation of somatic sensory nerves results in changes in autonomic functions [5,32]. The hypoglossal nucleus (HN) is the center of motor afferent control for lower jaw and tongue movements [8,21] and is involved with the taste and gustatory responses in the medulla [9,34,36]. The somatosensory information traveling along the limb nerves also modulates firing neurons in the HN [1,8,26]. However, the neural pathways and neurotransmitters responsible for anorexigenic effects of EA stimulation have not been studied in medulla areas.
It is well-documented that pro-opiomelanocortin (POMC), a precursor of an anorexigenic peptide melanocortin, plays a central role in the inhibitory regulation of appetite [6,7,10,41]. Activation of melanocortin in the NTS has been shown to result in a sustained reduction of food intake [12,19]. Previous studies from this laboratory have demonstrated that expression of neuronal nitric oxide (NO) synthase (nNOS) in the NTS and the GN is induced by EA stimulation of ST36 in rats [23]. Recent studies demonstrated that EA stimulation induced expression of the transient receptor potential vanilloid type-1 (TRPV1) endowed with nNOS immunostaining in subepidermal nerve fibers in the acupoints [13]. TRPV1 mediates sensory signal transduction [4,33,48], and TRPV1 has been demonstrated to contribute to feeding behavior and body weight signaling [35,42,46].
The purpose of this study is to determine the effects of advanced EA ST36, a two phase stimulation over 2 hrs, on changes in food intake and body weight in OP rats compared to obese resistant (OR) strain on a high fat diet. The mRNA levels of POMC, an anorexigenic peptide, were examined in the NTS, GN, and the HN regions of the rats after going through in vivo experimentation with and without advanced EA ST36. The influence of EA stimulation on the expression of signal cascades, nNOS and TRPV1, in the skin acupuncture point (acupoint, sensory afferents)-medulla pathway was also quantified and matched with its corresponding physiological data.
2. Materials and methods
2.1. Experimental animals
Obese Prone (OP) rats (7 week-old, 180 to 200 g) and Obese Resistant (OR) rats, their age-matched control strain, were acquired from Charles River (Wilmington MA). This outbred rat strain, previously called DIO, or Diet-Induced Obese rats [11,20,39], is specially bred to develop metabolic syndrome, despite having a functioning leptin receptor. The name was changed in order to avoid confusion with normal SD rats fed to obesity. The OR strain is otherwise similar to OP and outbred from the same line of rats, but is resistant to obesity even when fed a high fat diet. Using the OR strain as a control increases the likelihood that experimental differences are a result of obesity and not other factors. The OR and OP rats were fed a high fat diet (Purina Formulab Diet 5008) ad libitum for one week before the experiments and maintained a high fat diet throughout the duration of the experiments. The OP strain rapidly gained weight and developed metabolic syndrome, while the OR strain did not become overweight as was used as a control group [20,39]. The protocol was approved by the Harbor-UCLA Animal Care and Use Review Committee, and was in accord with AAALAC and NIH guidelines. The animals were maintained on a 12-hour light-dark cycle and in a humidity controlled room at a temperature 24±1C. Food pellets were weighed; food intake and body weight were recorded at 24 hours each.
2.2. Electroacupuncture treatment
One week after the diet-treatment, EA stimulation of Zusanli (ST36) was performed in OP and OR rats. The rats in the treated group were placed in the immobilization apparatus consisting of a plastic cylinder. The acupuncture needles (27-gauge sharpened stainless-steel) were inserted percutaneously into a depth of 4 mm at ST36 located at the depression below the knee from the anterior crest of the tibia as previously described [23]. A Grass S48 stimulator connected to a stimulus isolator 360A (WPI, Sarasota, FL) was connected to each pair of the acupuncture needles bilaterally in ST36. Advanced EA stimulation of ST36 is defined as using 1.0 mA, duration of 1.0 msec at 10 Hz for 20 min and conducted twice, separated by an 80 min rest interval in conscious rats [31]. The rats in OP sham EA group were placed in the immobilization apparatus consisting of a plastic cylinder, and needles were taped on the surface of acupoint ST36 without electrical stimulation. The rats in the control group, including OP and OR rats, were not given any stimulation. The treatments were performed on all groups simultaneously each day, lasting for 7 days. Food pellets were weighed; food intake and body weight were recorded at 24 hours each for 2 days before and 7 days post treatment. No animals showed any adverse signs or reactions to the treatments.
2.3. Tissue Preparation and RNA isolation
The OP rats at the end of the EA ST36 treatment and their age-matched control OR and OP control rats were sacrificed by decapitation. The brain and skin tissues were quickly removed and initially frozen on dry ice. The brain was then cut in 300-μm sections. The micropunches of the NTS and GN regions were identified and isolated bilaterally from two adjacent slices under a microscope by a punch made of a 18-gauge needle stub as described previously [2,22,27]. The brain nuclei were sampled, using coordinates NTS, AP-13.1, L 0–1.0, DV 6.0–7.0; GN, AP-14.3, L 0–1.0, DV 7.0–8.0. Acupoints on the skin together with subcutaneous tissue containing ST36 were isolated. Non-meridian skin tissue was obtained close to related acupoint but not in meridian area. Tissue samples of skin were immediately cut into slices (0.5 × 0.5 mm) with a tissue chopper. The samples of skin tissues and brain regions were isolated and frozen in liquid nitrogen and stored at −80 °C. Total RNA was isolated using Trizol Reagent (Invitrogen, USA) according to the manufacturer’s protocol. The amount of isolated total RNA was measured using spectrophotometry.
2.4. Real time PCR
Real time PCR was conducted using the RNA from each sample. Briefly, cDNA was reverse-transcribed from total RNA using a SuperScript ®, First-Strand Synthesis SuperMix for qRT-PCR System (Invitrogen, USA). Template cDNA was incubated with 200 nM gene specific primers of POMC, nNOS, TRPV1, and hypoxanthine-guanine phosphoribosyl transferase (HPRT, Applied Biosystems customer primer) respectively, and SYBR Green PCR Master Mix (Invitrogen, USA), and RNase-free water in a total reaction volume of 10 μl, performing in GeneAmp 7000 Sequence Detection System (Applied Biosystems, Foster City, CA). An initial step of 10 min at 95 °C activates the AmpliTaq, followed by 40 cycles of melting at 95°C for 15 s and annealing/extension at 60 °C for 1 min. The dissociation curve will be generated at the end of the PCR run by ramping the temperature of the sample from 60 to 90 °C. Meanwhile, the fluorescence data was continuously collected and shows a single product with a melting temperature (Tm) distinct from that of primer-dimer products. Non template controls were included for each primer pair to check for any significant levels of contaminants. The standard curve for each primer is constructed with different dilutions of cDNA. The amount of target mRNA of each sample was normalized with that of HPRT, an internal control. Oligonucleotide primers used for real-time RT-PCR analysis were designed based on the published sequences as follows: POMC [Forward: 5′-CAAGAAGCGGCGCCCTGTGA; Reverse: 5′-GCTGCT CGCCTTCCAGCTCC], nNOS [Forward: 5′-ACGTTTGGGGTTCAGCAGATCCAA; Reverse: 5′-GGGAGGCTTGCTGACCCGTT], TRPV1 [Forward: 5′-AGATGGGCATCTATGCTGTC; Reverse: 5′-TTCTTCCCATCCTCAATCAG], HPRT [Forward: 5′-CAAAGCCTAAAAGACAG CGG; Reverse: 5′-ATGGCCACAGGACTAGAACG].
2.5. Data presentation and statistical analysis
All data are shown as mean ± SEM to determine the significance between different groups. Four to six rats were used for each defined group. Analysis of variance (ANOVA and Post Hoc Tests) for comparisons among multiple groups, and Student’s t-test (unpaired) for comparisons when analysis of two groups is involved were used to determine the significance of differences. P values less than 0.05 were considered significant.
3. Results
3.1. Effects of acupuncture on food intake
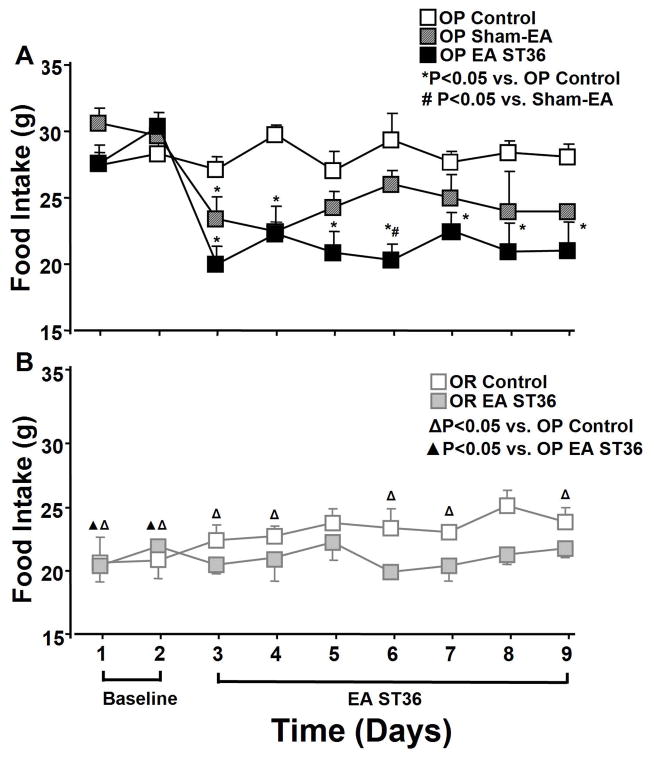
Food intake and body weight were recorded 2 days before as baseline and 7 days following treatments during the course of 9 days (Fig. 1). OP rats had a significantly higher baseline food intake (20%) compared to OR rats (F=7.074, P=0.007).
Figure 1.
Daily food intake starting 2 days before and 7 days during EA ST36 in conscious obesity-prone (OP) with high fat diet rats compared to sham EA group and a control group without treatment (A, top panel). Average daily food intake in the same period was also recorded in conscious obesity resistant (OR) rats with high fat diet following EA ST36 compared to a control group (B, bottom). ANOVA analysis revealed significant reduction of food intake in OP rats compared to the control rats (top) following EA ST36 in day 2 (F=8.676, P=0), day 3 (F=15.692, P=0), day 4 (F=4.918, P=0.002), day 5 (F=8.185, P=0), day 6 (F=5.43, P=0.002), day 7 (F=6.008, P=0.001, and day 8 (F=6.065, P=0.001), but not altered in OR rats (bottom). OP control group had significantly higher food intake than OR control rats. Values were expressed as mean ± SEM (n = 5–6 each group) regardless of the direction of the error bar on the figure. P<0.05 *vs. OP control group, # vs. sham-EA group, △ vs. OP control group, ▲ vs. OP EA treated group.
The food intake in EA treated OP rats significantly decreased 25 % after the first day of advanced EA ST36 and continued for the remaining treatment days (P<0.05; Fig. 1A). Sham EA group also produced significant reduction of food intake when compared to the OP control group for the first two days of the treatment (day 2 F=8.676, P=0.05, and day 3 F=15.692, P=0, n=6), as shown in figure 1A. Sham-EA tended to decrease in food intake compared to OP control group on the last day treatment although the difference was short of statistical significance (P=0.111). However, changes in food intake by sham EA in the remaining days was not statistically significant compared to the untreated OP rats. Over the 7 days of experimentation, OP rats with advanced EA ST36 averaged 21.1 ± 1.5 grams of food consumed per day, OP rats with sham EA averaged 24.1 ± 1.5 grams, and OP rats without any treatment averaged 28.2 ± 1.1 grams. The amount of food intake in OP treated rats with advanced EA ST36 was reduced to levels similar to that in OR treated rats, and slightly lower than OR control rats, as shown in figure 1. The values of food intake in OR rats following advanced EA ST36 tended to be decreased compared to that in control OR rats, but did not have statistical significance, as shown in figure 1B (n=4).
3.2. Effects of acupuncture on body weight
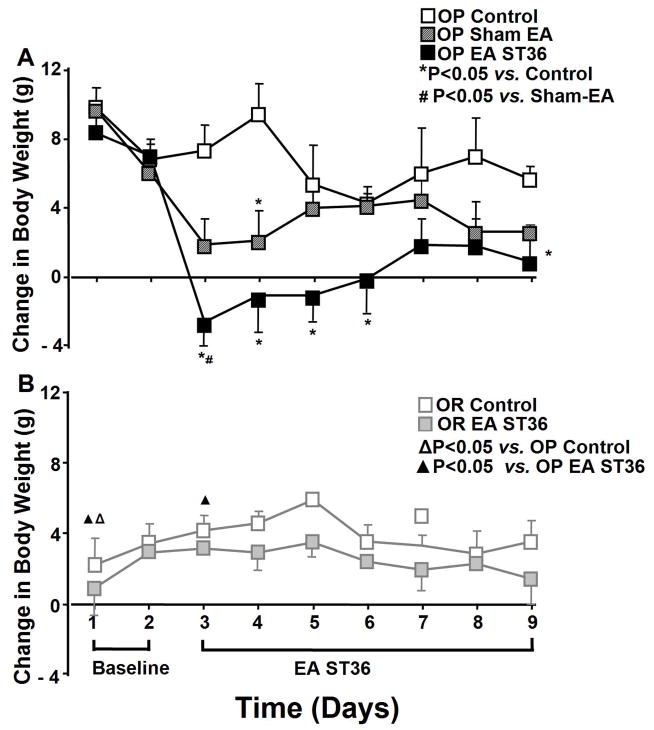
The baseline body weight and increases in the weight were significantly higher in OP rats versus in OR rats during the 9 days (P<0.05). Baseline body weight change was not significantly different among 9 days in OR control rats and OP control rats. The increases in body weight was 2–3 fold in OP control rats versus in OR control rats with high fat diet during the 9 days of experimentation, as shown in figure 2.
Figure 2.
Daily body weight change beginning 2 days before and 7 days following EA stimulation of ST36 in conscious obesity-prone (OP) rats compared to sham EA group and a control group without treatment (A, top panel). Average daily body weight change in the same period was also recorded in obesity resistant (OR) conscious rats with EA ST36 compared to a control group (B, bottom). Body weight changes were significantly reduced by EA ST36 in OP rats compared to the control rats without the treatment (top) on day 2 (F=17.144, P=0), day 3 (F=11.373, P=0), day 4 (F=2.936, P=0.012), day 5 (F=4.311, P=0.03), day 6 (F=0.804, P=0.161), day 7 (F=1.741, P=0.056), and day 8 (F=4.480, P=0.009), but not altered in OR rats (bottom). OP control group had a higher trend of body weight change than OR control rats, but did not show the significant difference except day 1. Other details are shown in legend to Figure 1.
Figure 2A shows body weight change in the untreated OP and sham EA groups compared to EA treated OP group. Body weight was significantly decreased in OP treated rats after day 1 of EA ST36 and continued for the remainder of the 7 treatment days compared to untreated OP control rats (n=6; P<0.05). The elevation of body weight was reduced in OP treated rats after day 1 of advanced EA ST36 and continued for the remainder of the 7 treatment days compared to untreated OP control rats. Body weight was significantly reduced by sham EA only on day 4 day (n=6) compared to untreated OP rats. Sham EA tended to decrease body weight on the last day (P=0.179) but the reduction failed to be statistically significant.
Figure 2B shows body weight change in the untreated OR rats compared to EA treated OR group at 2 days before as baseline and 7 days following EA ST36. The values of body weight change in OR rats were moderately decreased at 7 days following advanced EA ST36 compared to OR control rats, but the difference failed short of significance (n=4). When comparing EA treated OP rats with sham treated OP rats, the difference in body weight was significant only on day 3 (F=17.144, P=0.018, n=5).
3.3. Expression of POMC, nNOS, and TRPV1 mRNAs in medulla regions induced by EA
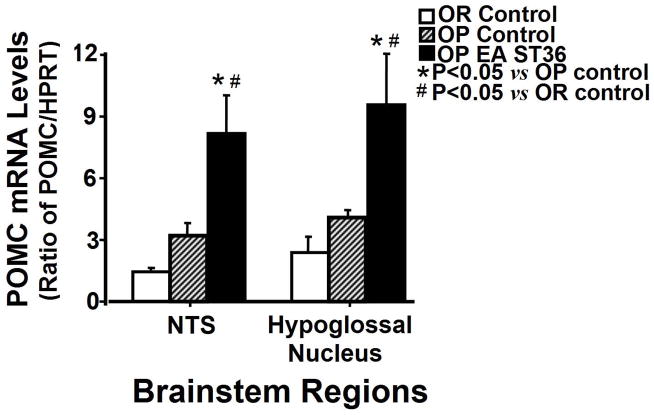
Figure 3 shows the influence of EA ST36 on the expression of POMC mRNA in the dorsal medulla regions of OP rats with advanced EA ST36 compared to OR and OP control rats. The expression of POMC mRNA was significantly increased about 2.5-fold by EA ST36 in the NTS region of OP rats compared to OP and OR control groups (Fig. 3, the left side; F=8.418, P=0.003). Simultaneously, the expression of POMC mRNA in the HN region of OP treated rats showed about 2.3-fold increase compared to OP and OR control rats (Fig. 3, the right side; F=5.653, p=0.011).
Figure 3.
Comparison of POMC mRNA levels in the brainstem regions from OP rats treated with EA ST 36, OP control rats, and OR control rats. Each bar represents the mean values of the relative amount of mRNAs as ratio between POMC and ypoxanthine-guanine phosphoribosyl transferase (HPRT), an internal standard control. Values were expressed as mean ± SEM (n = 4–5 each group). NTS, nucleus tractus solitarius; * P<0.05 indicates significance versus another group in the same region.
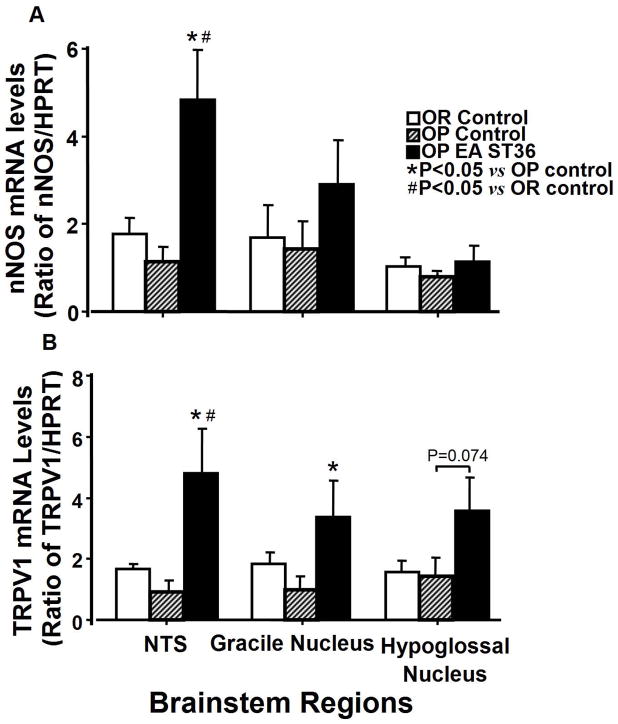
Figure 4 shows influences of EA ST36 on the expression of nNOS (top) and TRPV1 (bottom) mRNA in the dorsal medulla regions. nNOS mRNA expression in the NTS region of OP control rats tended to be lower than that in OR control rats but the difference failed short of significance. EA ST36 produced a marked increase in the expression of nNOS mRNA in the NTS region of OP rats compared to untreated OP and OR groups (Fig. 4A, the left side; F=3.132, P=0.002). EA ST36 produced an elevation of nNOS expression in the GN region of OP rats, but did not achieve statistical significance.
Figure 4.
Comparison of the expression of nNOS and TRPV1 mRNAs in the brainstem regions from OP rats treated with EA ST 36, OP control rats, and OR control rats (Fig. 4A). TRPV1 expression levels in the brainstem regions from OR rats compared to OP rats with and without EA stimulation to ST36 are shown in Figure 4B. Each bar represents the mean values of the relative amount of mRNAs as ratio between target gene and internal standard control, hypoxanthine-guanine phosphoribosyl transferase (HPRT). Other details are shown in legend to Figure 3.
EA ST36 in OP rats caused a marked increase in TRPV1 mRNA expression in the NTS region compared to OP and OR control rats (Fig. 4B, the left side; F=4.948, P=0.005). TRPV1 mRNA expression in the GN region was also significantly elevated by EA ST36 in OP rats compared to untreated OP and OR rats (Fig. 4B, the middle; F=2.006, p=0.051). The level of TRPV1 mRNA in the HN region of OP rats following EA stimulation suggested a marginal significant increase compared to OP and OR control rats (Fig. 4B right).
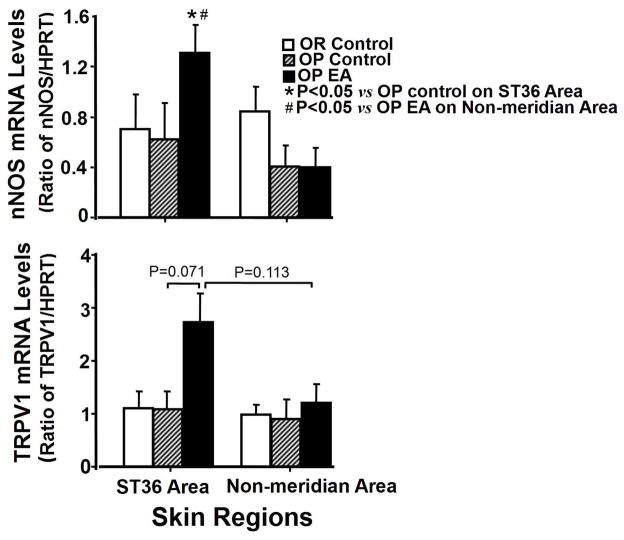
3.4. Expressions of nNOS and TRPV1 mRNA in the skin acupoint induced by EA
Figure 5 shows the influences of EA stimulation to ST36 on the expression of nNOS and mRNAs in ST36 region compared to non-meridian areas of the skin. EA treatment caused a significant increase in nNOS mRNA expression, approximately 2.1-fold in ST36 region from EA treated OP rats compared to an untreated OP control group, and about 3.3-fold compared to non-meridian region from OP treated rats (Fig. 5 top; F=2.679, P=0.049, P=0.007). EA ST36 induced a marginally significant increased expression of TRPV1 mRNA in ST36 region from OP EA treated rats compared to that from OP control group (F=1.215, P=0.071).
Figure 5.
Expression of nNOS mRNA (Fig. 5A) and TRPV1 mRNA (Fig. 5B) in the skin regions containing ST36 and non-meridian area from OP treated rats with EA ST 36, OP control rats, and OR control rats. Each bar represents the mean values of the relative amount of target mRNA versus HPRT as a control housekeeping gene. * P<0.05 indicates significance versus another group in the same region. Other details are shown in legend to Figure 3.
4. Discussion
We observed the effects of advanced EA ST36 (two phases over 2 hrs separated by an 80 min interval) on changes in food intake and body weight in OP rats and OR rats on a high fat diet. The influences of the EA treatment on the expression of an anorexigenic peptide POMC in the medulla regions and signal cascades, and of nNOS and TRPV1, in the afferents-medulla pathway, were also examined in OP rats. The major new findings from this study are: 1) Food intake and body weight were consistently reduced by advanced EA ST36 in OP rats; 2) POMC mRNA was markedly enhanced in the NTS and HN regions by advanced EA ST36 in OP rats; and 3) TRPV1 and nNOS mRNA levels were consistently increased in the skin region of ST36 and in the NTS and the GN regions by advanced EA ST36 in OP rats. This is the first evidence showing that the reduction of food intake and body weight by advanced EA ST36 in OP rats is associated with a marked increase in the POMC mRNA level in the NTS and HN regions. The experimental results demonstrate that elevated mRNA level of POMC in the dorsal medulla regions and reduced food intake and body weight are predominantly produced by advanced EA ST36 for 7 days in OP rats, but not in OP rats held in the same immobilization apparatus with sham-EA treatment. Advanced EA ST36 produced slight effects in reduction of food intake and body weight in OR rats. The present studies also show that advanced EA ST36 induces up-regulation of TRPV1 and nNOS mRNAs in both the local afferents, acupoint area, and the medulla sensory regions, the NTS, the GN, and the HN. Enhanced expressions of TRPV1 and nNOS mRNAs in the ST36 and the brainstem regions are consistently induced by EA ST36, which play a role in the signal transduction of EA stimulation from peripheral afferents to the dorsal medulla pathway. Results suggest that advanced EA ST36 stimulates up-regulation of POMC, an anorexigenic peptide, in the NTS and the HN, which inhibits feeding behavior and reduces body weight. EA-induced expression of the signal molecules, TRPV1-nNOS, via a pathway from the somatic skin to the sensory nuclei in the medulla, mediate the signal transduction of EA stimuli, which contribute to the mechanism of the therapy.
It has been shown that acupuncture and EA stimulation of the hindlimb acupoint Zusanli (ST36) produce effects in the treatment of obesity, particularly used in China for thousands of years [3,17,37,43]. However, recent clinical studies have shown that acupuncture has some effects, but not sufficiently strong, in decreasing body weight of obese subjects [17]. The effects induced by EA twice with a non-stimulation interval of 90 min following the first EA are markedly enhanced after the second EA. The therapeutic effects induced by EA presented twice in succession are markedly enhanced in the second EA at 90 min following the first EA [29]. It is believed that stronger effects of acupuncture need a prolonged induction time, in which it also has a delayed recovery period [24,29,31]. Thus, a two phase advanced EA treatment utilizing two stimulation periods over 2 hrs with is observed in this study. The present studies show that advanced EA ST36 produces a marked reduction of food intake and body weight in a diet-induced obese rat model. The increases in body weight was 2–3 fold in OP control rats versus in OR control rats with high fat diet during the 9 days of experimentation. Following day 1 of advanced EA ST36 and continued for the remainder of the 7 treatment days, food intake in OP rats was reduced to the amounts similar to OR control rats, and the elevation of body weight was revised in OP treated rats compared to untreated OP control rats. Whether this effect can be maintained over a longer period or repeated in humans remains to be seen by further investigation. It is also shown that the same immobilization apparatus of EA ST36 produced slight effects in reduction of food intake and body weight in OR rats, which suggest that more pronoce effects can be induced by acupuncture in rats with pathological changes.
It is well-documented that POMC is a precursor of melanocortin, which plays an important role in reduction of feeding activity [6,7,10,41]. Our results revealed that expression of POMC mRNA was increased in the NTS and HN by advanced EA ST36 in OP rats and accompanied by a reduction of food intake. These results are consistent with results from other investigators who have demonstrated that activation of POMC in the NTS results in a sustained reduction of food intake [12,19]. Previous studies have demonstrated that the HN is the center of motor afferent control for lower jaw and tongue movements [8,21] and is involved with the taste and gustatory responses in the medulla [9,34,36]. Ingestion and anticipation of a meal also created increased Fos expression in the HN [9]. The somatosensory information traveling along the forelimb nerves also modulates firing neurons in the HN, suggesting that somatosensory signals, together with visual messages, induce tongue reflex responses functionally directed to modulate the postural tone of the tongue and the oral cavity for food reception [1,8,25,26]. Our studies support the previous publications [1,8,9,21,26,34,36] reported that the HN plays an important role in feeding movements and in response to somatosensory activation. The results further suggest that EA ST36-induced expression of POMC mRNA in the GN is involved in reduction of food intake and body weight in OP rats. The present studies provide molecular and physiological evidence of anorexigenic peptide POMC in the NTS/HN-mediated appetitive inhibition and anti-obese effects of EA ST36.
Recent studies have shown that TRPV1 is expressed at the primary afferent neurons, which play an important role in mechanical, thermal, chemical, and many other sensory transductions [4,33,48]. The colocalization of TRPV1 and nNOS was previously reported in the neurons [16], and TRPV1 is activated by NO through a feedback regulation mechanism between channel activation, calcium entry and NO production [45], which suggests an important function of both TRPV1 and NO involved in the sensory transmission. Previous studies from our laboratory have reported that performing EA on ST36, a vital leg acupoint, increased positive immunostaining nNOS neurons in the NTS and the GN [23]. A high expression of TRPV1 endowed with nNOS in subepidermal nerve fibers exist in the acupoints, and its expression is increased by EA stimulation [13]. The present study’s findings are consistent with these results, demonstrating that EA ST36 significantly increased expression of the TRPV1 and nNOS mRNAs in ST36 regions and in the NTS and GN. The higher expression of TRPV1 and nNOS in the ST36 acupoint after EA stimulation may play a key role in mediating the transduction of EA signals to the dorsal medulla. In addition, TRPV1 activation of sensory neurons modified feeding behavior [35,46] and reduced short term food intake [42]. The oral administration of capsaicin for 120 days prevented obesity in male wild type mice but not in TRPV1 knockout mice assigned to high fat diet, and other in vitro and in vivo evidences suggested the activation of TRPV1 channels by capsaicin prevented adipogenesis and obesity [47]. Since activation of TRPV1 plays a role in the reduction of food intake [42,47], it is possible that EA-induced elevation of TRPV1 expression in the NTS and the GN might affect NTS (possibly also the GN) neurons directly to regulate feeding behavior. Although the mechanism responsible for inhibition of food intake is not entirely understood, increased TRPV1-nNOS levels post-EA stimulation also suggests a functional role in mediating the effects of EA through the peripheral site to the central nervous system. A more sophisticated approach would be required to address this issue. Despite these limitations, our results consistently suggest that NO-TRPV1 mediate sensory transduction of EA signaling through somatosensory afferents (acupoints)-dorsal medulla pathway.
In summary, our results show that advanced EA ST36 decreases food intake and reduces body weight in OP rats, but produced slight effects in OR rats. Consistent with these observations, mRNA level of POMC was markedly enhanced in the NTS and HN regions following the EA treatment. Advanced EA ST36 also induces an elevation of nNOS and TRPV1 mRNAs in the NTS and GN regions, the sensory nuclei entering the brainstem, accompanied by increases in the levels of nNOS and TRPV1 expression in the skin region of acupoint ST36. The results suggest that advanced EA ST36 stimulates up-regulation of TRPV1-nNOS in the peripheral afferents and sensory nuclei in the medulla, which mediate the signal transduction of EA stimuli to enhance POMC production in the NTS/HN, and in turn, inhibits feeding behavior and reduces body weight. The evidence in OP rats demonstrates that advanced EA ST36 may be used to alleviate hyperphagia and obesity in metabolic syndrome.
Highlights.
Food intake and body weight were reduced by acupuncture ST36 in Obese Prone rats
Acupuncture ST36 increased POMC mRNA level in NTS and hypoglossal nucleus regions.
TRPV1 and nNOS mRNAs were increased by acupuncture ST36 in the NTS/GN regions.
Acupuncture ST36 increased TRPV1 and nNOS mRNAs in the skin acupoints.
Acknowledgments
Funding:
This project was made possible by NIH Grants (AT004504, and AT004620) from the National Center for Complementary and Alternative Medicine to Dr. Ma.
These studies were conducted at the biomedical research facilities of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center. We are grateful to Drs. Brian Smith, Peng Xia, and Zhichun Jiang, who provided excellent technical support.
List of abbreviations
- Acupoint
Acupuncture point
- EA
Electroactupuncture
- OP
Obese Prone
- OR
Obese Resistant
- TRPV1
Transient Potential Vannilloid 1
- nNOS
neuronal Nitric Oxide Synthase
- POMC
Pro-opiomelanocortin
- NTS
Nucleus Tractus Solitarius
- GN
Gracile Nucleus
- HN
Hypoglossal Nucleus
- ST36
Zusanli
Footnotes
Conflict of interest
All authors in the manuscript declare no conflicts of interests.
Author Contributions:
Dr. Sheng-Xing Ma takes responsibility for the entire project including study concept, research design, study supervision, and obtained funding from the National Center for Complementary and Alternative Medicine. Conduct of the study, acquisition and analysis of data: Dr. Bo Ji and Jay Hu. All authors contributed to manuscript preparation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Borke RC, Nau ME, Ringler RL., Jr Brain stem afferents of hypoglossal neurons in the rat. Brain Res. 1983;269:47–55. doi: 10.1016/0006-8993(83)90961-7. [DOI] [PubMed] [Google Scholar]
- 2.Brudzynski SM, Wu M, Mogenson GJ. Decreases in rat locomotor activity as a result of changes in synaptic transmission to neurons within the mesencephalic locomotor region. Can J Physiol Pharmacol. 1993;71:394–406. doi: 10.1139/y93-060. [DOI] [PubMed] [Google Scholar]
- 3.Cabioglu MT, Gündogan N, Ergene N. The efficacy of electroacupuncture therapy for weight loss changes plasma lipoprotein A, apolipoprotein A and apolipoprotein B levels in obese women. Am J Chin Med. 2008;36:1029–1039. doi: 10.1142/S0192415X08006430. [DOI] [PubMed] [Google Scholar]
- 4.Cioffi DL. The Skinny on TRPV1. Circ Res. 2007;100:934–936. doi: 10.1161/01.RES.0000265139.10277.62. [DOI] [PubMed] [Google Scholar]
- 5.Cliffer KD, Hasegawa T, Willis WD. Responses of neurons in the gracile nucleus of cats to innocuous and noxious stimuli:basic characterization and antidromic activation from the thalamus. J Neurophysiol. 1992;68:818–832. doi: 10.1152/jn.1992.68.3.818. [DOI] [PubMed] [Google Scholar]
- 6.Cone RD. Anatomy and Regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 7.Cone RD. The central melanocortin system and energy homeostasis. Trends Endocrinol Metab. 1999;10:571–578. doi: 10.1016/s1043-2760(99)00153-8. [DOI] [PubMed] [Google Scholar]
- 8.Elmund J, Bowman JP, Morgan RJ. Vestibular influence on tongue activity. Exp Neurol. 1983;81:126–140. doi: 10.1016/0014-4886(83)90162-0. [DOI] [PubMed] [Google Scholar]
- 9.Emond MH, Weingarten HP. Fos-like immunoreactivity in vagal and hypoglossal nuclei in different feeding states: a quantitative study. Physiol Behav. 1995;58:459–465. doi: 10.1016/0031-9384(95)00069-u. [DOI] [PubMed] [Google Scholar]
- 10.Fan W, Boston BA, Kesteron RA, Gruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agoutic obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 11.Geiger BM, Behr GG, Frank LE, Caldera-Siu AD, Beinfeld MC, Kokkotou EG, Pothos EN. Evidence for defective mesolimbic dopamine exocytosis in obesity-prone rats. Faseb J. 2008;22:2740–2746. doi: 10.1096/fj.08-110759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grill HJ, Ginsberg AB, Seeley RJ, Kaplan JM. Brainstem application of melanocortin receptor ligands produces long-lasting effects on feeding and body weight. J Neurosci. 1998;18:10128–35. doi: 10.1523/JNEUROSCI.18-23-10128.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibrahim TS, Chen ML, Ma SX. TRPV1 expression in acupuncture points: Response to electroacupuncture stimulation. J Chem Neuroanat. 2011;41:129–136. doi: 10.1016/j.jchemneu.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeffery RW, Drewnowski A, Epstein LH, Stunkard AJ, Wilson GT, Wing RR, Hill DR. Long-term maintenance of weight loss: current status. Health Psychol. 2000;19:5–16. doi: 10.1037/0278-6133.19.suppl1.5. [DOI] [PubMed] [Google Scholar]
- 15.Joseph SA, Pilcher WH, Bennett-Clarke C. Immunocytochemical localization of ACTH perikarya in nucleus tractus solitarius: evidence for a second opiocortin neuronal system. Neurosci Lett. 1983;38:221–225. doi: 10.1016/0304-3940(83)90372-5. [DOI] [PubMed] [Google Scholar]
- 16.Koike S, Uno T, Bamba H, Shibata T, Okano H, Hisa Y. Distribution of vanilloid receptors in the rat laryngeal innervation. Acta Otolaryngol. 2004;124:515–519. doi: 10.1080/00016480310000674. [DOI] [PubMed] [Google Scholar]
- 17.Lacey JM, Tershakovec AM, Foster GD. Acupuncture for the treatment of obesity: a review of the evidence. Int J Obes Relat Metab Disord. 2003;27:419–27. doi: 10.1038/sj.ijo.0802254. [DOI] [PubMed] [Google Scholar]
- 18.Leem JW, Lee BH, Willis WD, Chung JM. Grouping of somatosensory neurons in the spinal cord and the gracile nucleus of the rat by cluster analysis. J Neurophysiol. 1994;72:2590–2597. doi: 10.1152/jn.1994.72.6.2590. [DOI] [PubMed] [Google Scholar]
- 19.Li G, Zhang Y, Rodrigues E, Zheng D, Matheny M, Cheng KY, et al. Melanocortin activation of nucleus of the solitary tract avoids anorectic tachyphylaxis and induces prolonged weight loss. Am J Physiol Endocrinol Metab. 2007;293:252–258. doi: 10.1152/ajpendo.00451.2006. [DOI] [PubMed] [Google Scholar]
- 20.Li S, Zhang HY, Hu CC, Lawrence F, Gallagher KE, Surapaneni A, Estrem ST, Calley JN, Varga G, Dow ER, Chen Y. Assessment of diet-induced obese rats as an obesity model by comparative functional genomics. Obesity. 2008;16:811–818. doi: 10.1038/oby.2007.116. [DOI] [PubMed] [Google Scholar]
- 21.Lorier AR, Funk GD, Greer JJ. Opiate-induced suppression of rat hypoglossal motoneuron activity and its reversal by ampakine therapy. PLoS One. 2010;5:e8766. doi: 10.1371/journal.pone.0008766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma SX, Ignarro LJ, Byrns R, Li XY. Increased nitric oxide production in posterior hypothalamus and central sympathetic function on arterial pressure tolerance to nitroglycerin in rats. Nitric Oxide Biol Chem. 1997;3:153–161. doi: 10.1006/niox.1999.0218. [DOI] [PubMed] [Google Scholar]
- 23.Ma SX, Ma J, Moise G, Li XY. Responses of neuronal nitric oxide synthase expression in the brainstem to electroacupuncture Zusanli (ST 36) in rats. Brain Res. 2005;10:70–77. doi: 10.1016/j.brainres.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 24.Ma SX. Neurobiology of acupuncture: Toward CAM. Evid Based Complement Alternat Med. 2004;1:41–47. doi: 10.1093/ecam/neh017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006;84:274–288. doi: 10.1093/ajcn/84.1.274. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mameli O, Tolu E. Somatosensory input from the forelimb nerves to the hypoglossal neurons. Exp Neurol. 1986;94:757–766. doi: 10.1016/0014-4886(86)90253-0. [DOI] [PubMed] [Google Scholar]
- 27.Miyamae T, Goshima Y, Yue JL, Misu Y. L-dopaergic components in the caudal ventrolateral medulla in baroreflex neurotransmission. Neurosci. 1999;92:137–149. doi: 10.1016/s0306-4522(98)00721-0. [DOI] [PubMed] [Google Scholar]
- 28.Morrill AC, Chinn CD. The obesity epidemic in the United States. J Public Health Policy. 2004;25:353–366. doi: 10.1057/palgrave.jphp.3190035. [DOI] [PubMed] [Google Scholar]
- 29.Pomeranz B, Warma N. Electroacupuncture suppression of a nociceptive reflex is potentiated by two repeated electroacupuncture treatments: The first opioid effect potentiates a second non-opioid effect. Brain Res. 1988;452:232–236. doi: 10.1016/0006-8993(88)90028-5. [DOI] [PubMed] [Google Scholar]
- 30.Riva G, Bacchetta M, Cesa G, Conti S, Castelnuovo G, Mantovani F, Molinari E. Is severe obesity a form of addiction? Rationale, clinical approach, and controlled clinical trial. Cyberpsychol Behav. 2006;9:457–479. doi: 10.1089/cpb.2006.9.457. [DOI] [PubMed] [Google Scholar]
- 31.Rong PJ, Ma SX. Electroacupuncture Zusanli (ST36) on release of nitric oxide in the gracile nucleus and improvement of sensory neuropathies in Zucker Diabetic Fatty rats. Evid Based Complement Alternat Med. 2009 Aug 13;:1–11. doi: 10.1093/ecam/nep103. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samso E, Farber NE, Kampine JP, Schmeling WT. The effects of halothane on pressor and depressor responses elicited via the somatosympathetic reflex: A potential antinociceptive action. Anesth Analg. 1994;79:971–979. doi: 10.1213/00000539-199411000-00026. [DOI] [PubMed] [Google Scholar]
- 33.Stander S, Moormann C, Schumacher M, Buddenkotte J, Artuc M, Shpacovitch V, Brzoska T, Lippert U, Henz BM, Luger TA, Metze D, Steinhoff M. Expression of vanilloid receptor subtype 1 in cutaneous sensory nerve fibers, mast cells and epithelial cells of appendage structures. Exp Dermatol. 2004;13:129–139. doi: 10.1111/j.0906-6705.2004.0178.x. [DOI] [PubMed] [Google Scholar]
- 34.Streefland C, Jansen K. Intramedullary projections of the rostral nucleus of the solitary tract in the rat: Gustatory influences on autonomic output. Chem Senses. 1999;24:655–664. doi: 10.1093/chemse/24.6.655. [DOI] [PubMed] [Google Scholar]
- 35.Suri A, Szallasi A. The emerging role of TRPV1 in diabetes and obesity. Trends Phar Sci. 2007;29:29–36. doi: 10.1016/j.tips.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 36.Swank MW, Bernstein IL. C-Fos induction in response to a conditioned stimulus after single trial taste aversion learning. Brain Res. 1994;636:202–208. doi: 10.1016/0006-8993(94)91018-9. [DOI] [PubMed] [Google Scholar]
- 37.Tian DR, Li XD, Wang F, Niu DB, He QH, Li YS, Chang JK, Yang J, Han JS. Up-regulation of the expression of cocaine and amphetamine-regulated transcript peptide by electroacupuncture in the arcuate nucleus of diet-induced obese rats. Neurosci Lett. 2005;383:17–21. doi: 10.1016/j.neulet.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 38.Toney GM, Mifflin SW. Sensory modalities conveyed in the hindlimb somatic afferent input to nucleus tractus solitarius. J Appl Physiol. 2000;88:2062–2073. doi: 10.1152/jappl.2000.88.6.2062. [DOI] [PubMed] [Google Scholar]
- 39.Tschöp M, Heiman ML. Rodent obesity models: an overview. Exp Clin Endocrinol Diabetes. 2001;109:307–319. doi: 10.1055/s-2001-17297. [DOI] [PubMed] [Google Scholar]
- 40.Ueyama T, Houtani T, Ikeda M, Sato K, Sugimoto T, Mizuno N. Distribution of primary afferent fibers projecting from hindlimb cutaneous nerves to the medulla oblongata in the cat and rat. J Comp Neurol. 1994;341:145–158. doi: 10.1002/cne.903410202. [DOI] [PubMed] [Google Scholar]
- 41.Wang JH, Wang F, Yang MJ, Yu DF, Wu WN, Liu J, et al. Leptin regulated calcium channels of neuropeptide Y and proopiomelanocortin neurons by activation of different signal pathways. Neuroscience. 2008;156:89–98. doi: 10.1016/j.neuroscience.2008.04.079. [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Miares RL, Ahern GP. Oleoylethanolamide excites vagal sensory neurons, induces visceral pain and reduces short term food intake in mice via capsaicin receptor TRPV1. J Physiol. 2005;564:541–547. doi: 10.1113/jphysiol.2004.081844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wozniak P, Stachowiak G, Piêta-Doliñska A, Oszukowski P. Laser acupuncture and low-calorie diet during visceral obesity therapy after menopause. Acta Obstet Gynecol Scand. 2003;82:69–73. doi: 10.1034/j.1600-0412.2003.820113.x. [DOI] [PubMed] [Google Scholar]
- 44.Yin LL, Wang SX, Li YH. Advances of clinical studies on acupuncture and moxibustion for treatment of obesity in recent 10 years. Chin Acup Moxi. 2005;25:517–519. [PubMed] [Google Scholar]
- 45.Yoshida T, Inoue R, Morii T, Takahashi N, Yamamoto S, Hara Y, Tominaga M, Shimizu S, Sato Y, Mori Y. Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat Chem Biol. 2006;2:596–607. doi: 10.1038/nchembio821. [DOI] [PubMed] [Google Scholar]
- 46.Zhang LL, Liu DY, Ma LQ, et al. Activation of transient receptor potential vanilloid type 1 channel prevents adipogenisis and obesity. Circ Res. 2007;100:1063–1070. doi: 10.1161/01.RES.0000262653.84850.8b. [DOI] [PubMed] [Google Scholar]
- 47.Zhang LL, Yan LD, Ma LQ, Luo ZD, Cao TB, Zong J, Yan ZC, Wang LJ, Zhao ZG, Zhu SJ, Schrader M, Thilo F, Zhu ZM, Tepel M. Activation of transient receptor potential vanilloid type-1 channel prevents adipogenisis and obesity. Circ Res. 2007;100:1063–1070. doi: 10.1161/01.RES.0000262653.84850.8b. [DOI] [PubMed] [Google Scholar]
- 48.Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Juluis D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1997;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]