Fig. 1.
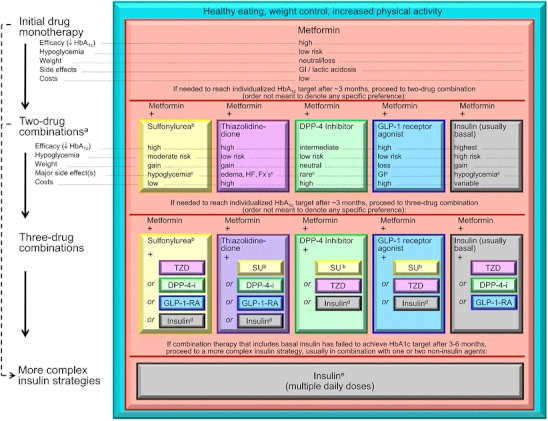
EASD/ADA 2012 Consensus statement. DPP-4-i, DPP-4 inhibitor; Fx’s, bone fractures; GI, gastrointestinal; GLP-1-RA, GLP-1 receptor agonist; HF, heart failure; SU, sulfonylurea. a, Consider beginning at this stage in patients with very high HbA1c (eg, ≥ 9 %). b, Consider rapid-acting, nonsulfonylurea secretagogues (meglitinides) in patients with irregular meal schedules or who develop late postprandial hypoglycemia on sulfonylureas. c, See Table 1 of the consensus statement for additional potential adverse effects and risks, under “Disadvantages.” d, Usually a basal insulin (NPH, glargine, detemir) in combination with noninsulin agents. e, Certain noninsulin agents may be continued with insulin (see text). Consider beginning at this stage if patient presents with severe hyperglycemia (≥16.7–19.4 mmol/L [≥300–350 mg/dL]; HbA1c ≥10.0 %–12.0 %) with or without catabolic features (weight loss, ketosis, etc). (With permission from: Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(6):1364–79. [48••]
