Figure 1.
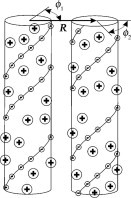
Model of electrostatic interaction between two parallel, double-stranded nucleic acids at close separations. The molecules are represented by dielectric cylinders with helical strands of point-like negative charges at the surface (small circles with the minus sign connected by helical lines). Bound counterions are modeled as point-like charges (large circles with the plus sign) at the cylinder surface, located randomly or in the middle between the strands of negative charges. Counterions responsible for the nonlinear screening of the nucleic acid charge are considered to be bound (7). Roughness of nucleic acid surfaces and poorly known dielectric properties of water in their grooves preclude evaluation of the effective cylinder radius with better than 2–3 Å accuracy from known molecular structures. For B-DNA, the effective radius that provides the best fit for the measured intermolecular forces is 11.2 Å, which is within the expected range between the 9 Å radius at centers and 12 Å radius at outer surfaces of phosphate groups (20). Because radii at the centers of phosphate groups in dsRNA and tsDNA are within 0.5 Å of that for B-DNA (Table 1), we use the same 11.2 Å effective radius for all three nucleic acids. It is important to emphasize that separation between nucleic acid surfaces in aggregates condensed by counterions is typically <10 Å (8,9), which is smaller than the separation between the strands of negative charges (Table 1). Therefore, it is essential to account for the discreteness and helical arrangement of the strands in any theory of such aggregates (7). Zipper-like juxtaposition of positively charged counterions bound in the grooves with negatively charged phosphate strands on the opposing molecule leads to electrostatic zipper attraction between the molecules, provided that bound counterions balance a sufficient fraction of the phosphate charges (14).
