Abstract
Background:
The growth in the production of biodiesel, which is principally fatty acid methyl esters (FAME), has been phenomenal in the last ten years because of the general desire to cut down on the release of greenhouse gases into the atmosphere, and also as a result of the increasing cost of fossil fuels.
Objective:
Establish whether there is any relationship between two different species (watermelon and muskmelon) within the same family (Cucurbitaceae) on fatty acid compositions and enumerate the different fatty acids in the two species.
Materials and Methods:
Extraction of fatty acids from the two species and preparation the extract to gas chromatography/mass spectroscopy analysis to determine the fatty acids compositions qualitatively and quantitatively.
Results:
The analyzed plants (watermelon and muskmelon) contain five saturated fatty acids; tetrdecanoic acid, pentadecanoic acid, hexadecanoic acid, heptadecanoic acid and octadecanoic acid with different concentrations, while muskmelon contains an extra saturated fatty acid named eicosanoic acid. The watermelon plant contains five unsaturated fatty acids while muskmelon contains three only, the two plants share in two unsaturated fatty acids named 9-hexadecenoic acid and 9-octadecenoic acid, the muskmelon plant contains higher amounts of these two acids (2.04% and 10.12%, respectively) over watermelon plant (0.88% and 0.25%, respectively).
Conclusion:
The chemical analysis of watermelon and muskmelon revealed that they are similar in saturated fatty acids but differ in unsaturated fatty acids which may be a criterion of differentiation between the two plants.
Keywords: Citrullus lanatus, Cucumis melo, fatty acids, gas chromatography/mass spectroscopy
INTRODUCTION
The growth in the production of biodiesel, which is principally fatty acid methyl esters (FAME), has been phenomenal in the last ten years because of the general desire to cut down on the release of greenhouse gases into the atmosphere, and also as a result of the increasing cost of fossil fuels. Thus vegetable oil producing countries are under pressure to expand production to meet the ever increasing demand. At the same time there is also concern about the strain on the world's supply of vegetable oils for food uses as more vegetable oils resources are diverted towards production of biodiesel.[1]
Watermelon (Citrullus lanatus) is taxonomically classified as a member of the Cucurbitaceae family, which is also known as the gourd family. Other gourds include pumpkins, cucumbers, squash, and other melons. It prefers warm climate growing conditions and is produced worldwide where conditions permit. Watermelon seeds are consumed as snack food worldwide and are used to prepare edible oil in some countries. Watermelon seed oil was prepared and evaluated for its physicochemical properties.[2,3] The seed oil consisted of 59.6% linoleic acid (18:2n-6) and 78.4% total unsaturated fatty acids. The predominant fatty acid in the oil was linoleic acid, which was followed by oleic, palmitic, and stearic acids.
Linolenic, palmitoleic, and myristic acids were minor constituents. The refractive index, acid value, peroxide value, and free fatty acids of watermelon seed oil were determined to be 1.4696 (25°C), 2.82 (mgKOH/g oil), 3.40 (mequiv oxygen/kg oil), and 1.41 (% as oleic acid), respectively. The saponification value of watermelon seed oil was 201 (mg KOH/g oil), and its iodine value was 115 (g iodine/100-g oil), which was significantly higher than pumpkin at 109 (g iodine/100-g oil).[2,3]
Muskmelon, Cucumis melo, is a member of the Cucurbitaceae family and grows best in tropical regions. The pulp of the fruit has pleasant flavor and taste, and the seeds are generally treated as waste; however, medicinal effects have been reported for the seeds.[4,5] Hexane-extracted seed oil of Cucumis melo hybrid AF-522 was determined to contain 64g of linoleic acid per 100g of total fatty acids.[4] Significant amounts of oleic, palmitic, and stearic acids were also detected in the melon seed oil. Earlier in 1986, the oil extracted from Cucumis melo seeds and examined its physicochemical properties.[5]
Total cholesterol and low density lipoprotein (LDL, “bad cholesterol”) concentrations were similar with palmitoleic and palmitic acids and significantly higher than with oleic acid.[6] High density lipoprotein (HDL, “good cholesterol”) was significantly lower with almitoleic than with palmitic acid. The study provides evidence that, at least in males with high blood cholesterol, a modest increase in palmitic acid raises LDL cholesterol relative to oleic acid, even when dietary cholesterol is low. Palmitoleic acid behaves like a saturated and not a monounsaturated fatty acid in its effect on LDL cholesterol. This work is consistent with previous observations that palmitoleic acid, among other fatty acids available in the diet, may be used by enzymes affecting fat oxidation.[7]
In this study, we aimed to establish whether there is any relationship between two different species (watermelon and muskmelon) within the same family (Cucurbitaceae) on fatty acid compositions and enumerate the different fatty acids in the two species.
MATERIALS AND METHODS
Collection of plant materials
The whole plant materials of watermelon (Citrullus lanatus (Thunb.), muskmelon (Cucumis melo) were collected from the private fields in Assfan village (60 km East Jeddah). The plant materials were ground with blender to fine powder. The samples collected early summer 2011 (April 2011) and identified through reference samples in the herbarium.
Extraction of oils
The plant samples of watermelon (Citrullus lanatus), muskmelon (Cucumis melo) used in this study were ground in a grinder in the presence of anhydrous sodium sulphate and accurately weighed. The ground plant materials (approximately 10g for each) were macerated with 300 ml of n-hexane at room temperature for 2 days, the macerates were shaken occasionally. Following filtration of the organic phases, the n-hexane phases of each sample was concentrated in vacuo at 40°C to obtain the oily residue.
Methyl esterification of the fatty acids
Three replicates comprising of healthy looking plants were analyzed. The oils were independently placed in 25 ml of volumetric flasks, then saponified by adding 12 ml 0.5 N methanolic sodium hydroxide to each mixture, and were heated on a steam bath until the fat globules disappeared. 2 ml of BF3/MeOH was added to each flask and the mixtures were boiled for 2 min. After the solutions were cooled down at room temperature, they were filled up to 25 ml with saturated sodium chloride solution and the fatty acid methyl esters (FAMEs) were prepared for each sample.[8] The obtained FAMEs were dissolved in n-hexane and 1 μl of samples was injected and analyzed by gas chromatography/mass spectroscopy (GC-MS).
Gas chromatography/mass spectroscopy analysis conditions
Chromatographic analysis by GC-MS analysis was carried out on Agilent HP 6890 Series combined with Agilent HP 5973 Mass Selective Detector (GC-MS). The capillary column used was Thermo Scientific (TR-5MS Capillary; 30.0 m × 0.25 mm ID × 0.25 μm film). Helium was used as carrier gas at a flow rate of 1.0 ml/min with 1 μl injection volume. Samples were analyzed with the column held initially at 140°C for 1 min after injection, then increased to 200°C with 5°C/min heating ramp with 3.0 min hold and increased to 215°C with 5°C/min heating ramp for 5 min. Then final temperature was increased to 240°C with 10°C/min heating ramp for 10.5 min. The injection was performed in split mode (20:1) at 270 °C. Detector and injector temperatures were 220°C and 200°C, respectively. Run time was 35 min. MS scan range was (m/z): 35-450 atomic mass units (AMU) under electron impact (EI) ionization (70 eV).
Identification of the fatty acids
Fatty acid components of the oils of the two plants were determined by comparing their retention times to authentic fatty acid samples obtained by GC and mass fragmentations with those of mass spectra database search (Wiley 7 n.1 and PMW_Tox 3.1).
RESULTS
In our study, the fatty acid profiles of the two vegetative plants (watermelon and muskmelon) samples. Saturated and unsaturated fatty acids and non-fatty acid compounds in the two plants were detected by GC analysis and molecular ion (m/z) and identification of these compounds were determined using Mass Specrtroscopy (MS).
The saturated fatty acids composition of watermelon plant were tetradecanoic acid, pentadecanoic acid, hexadecanoic acid, heptdecanoic acid and octadecanoic acid with retention times 7.28, 8.85, 10.02, 12.00 and 13.60 min, respectively [Table 1, Figures 1a–4]; while the saturated fatty acid derivatives were 5, 9, 13-trimethyl tetradecanoic acid, 2-methy octadecanoic acid, 2-hexyl cyclopropane octanoic acid and 3-pentyl oxirane undecanoic acid with retention times 9.56, 11.62, 11.90 and 12.48 [Figure 4]. The most abundant fatty acid was hexadecanoic acid with concentration percentage 48.36%, followed by octadecanoic acid with concentration percentage 12.73% [Figures 2 and 4]. The other fatty acids were minor in concentrations [Table 1].
Table 1.
Saturated fatty acids and their derivatives composition of Citrullus vulgaris (watermelon) and Cucumis melo (Muskmelon) detected by gas chromatography/mass spectroscopy
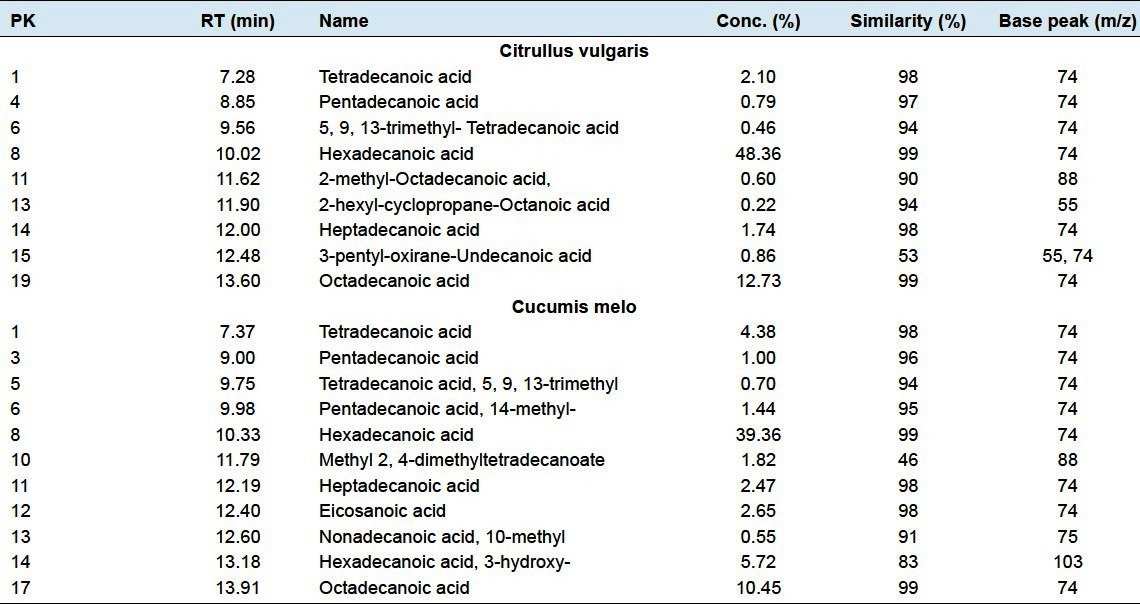
Figure 1.
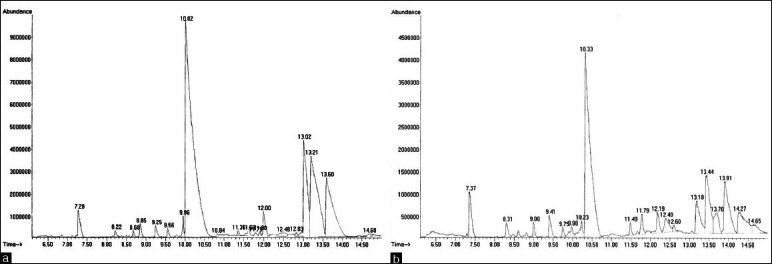
Gas chromatography profile for the extract of two the plants Citrullus vulgaris (watermelon) (a) and Cucumis melo (Muskmelon) (b)
Figure 4.
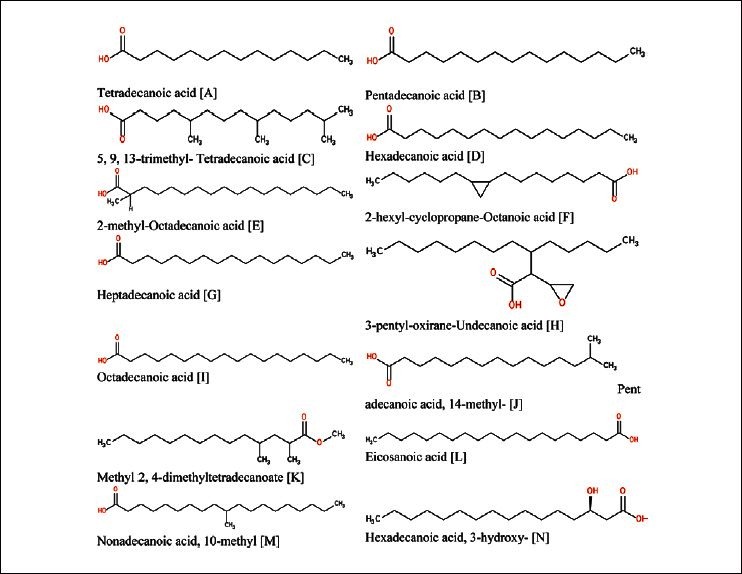
Fourteen saturated fatty acids found in the two plants Citrullus vulgaris (watermelon) and Cucumis melo (Muskmelon)
Figure 2.
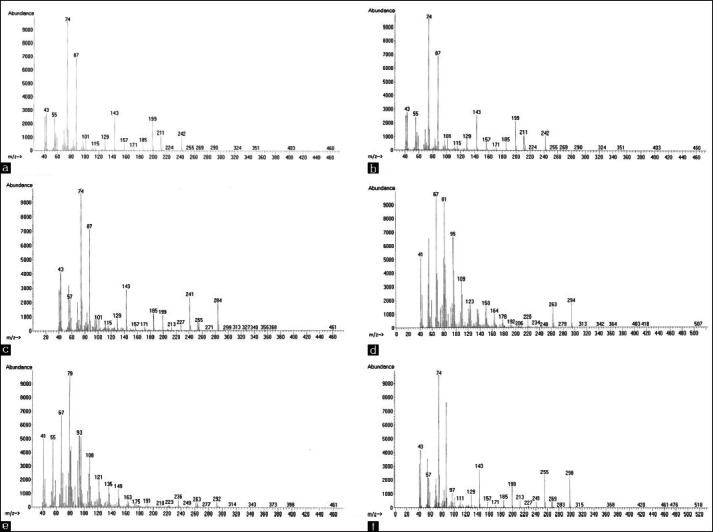
Mass spectra of the most abundant fatty acids in extracts of Citrullus vulgaris (watermelon) plant. (a) Tetradecanoic acid, (b) Hexadecanoic acid, (c) Heptadecanoic acid, (d) 8,11-Octadecadienoic acid, (e) 9,12,15-Octadecatrienoic acid and (f) Octadecanoic acid
The watermelon plant contains five unsaturated fatty acids called 9-hexadecenoic acid, 9-octadecenoic acid, 9,12-octadecadienoic acid (Z,Z), 8, 11-octadecadienoic acid and 9,12,15-octadecatrienoic acid at 9.96, 10.8411.78, 13.02 and 13.21 min retention times, respectively [Table 2]. The most abundant unsaturated fatty acid was 9,12,15-octadecatrienoic acid with concentration percentage 17.48%, followed by 8,11-octadecadienoic acid with 10.76% concentration [Figures 2 and 5].
Table 2.
Unsaturated fatty acids and their derivatives composition of Citrullus vulgaris (watermelon) and Cucumis melo (Muskmelon) detected by gas chromatography/mass spectroscopy
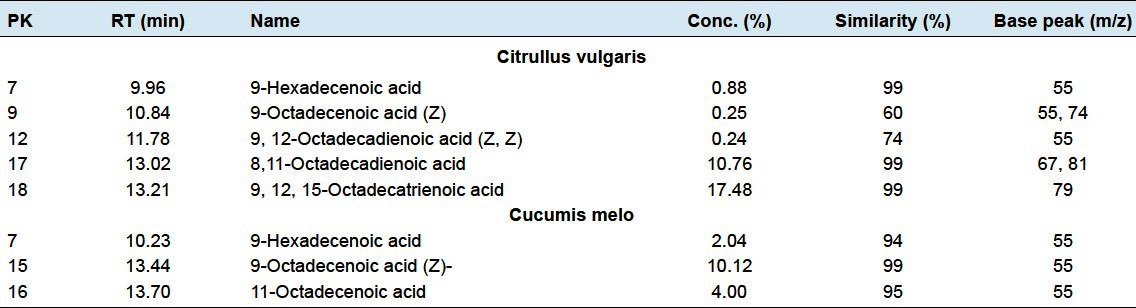
Figure 5.
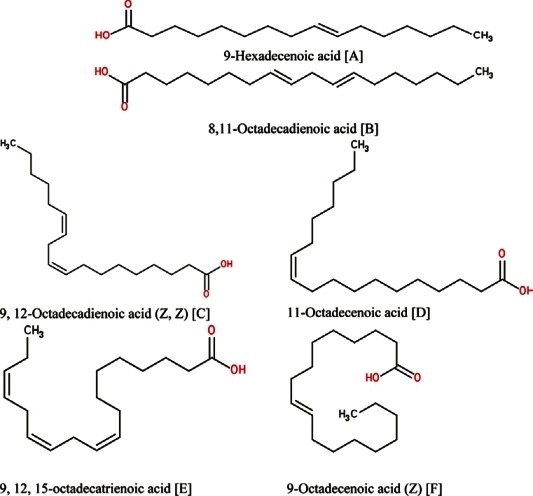
Six unsaturated fatty acids found in the two plants Citrullus vulgaris (watermelon) and Cucumis melo (Muskmelon)
There are 6 non-fatty acid compounds 1-octadecene, 8-hexadecen-1-ol acetate (Z), 6, 10,14-trimethyl-2-pentadecanone, 1-heptadecene, 4,6-dimethyl-undecane and 6-heptyltetrahydro-2H-pyran-2-one, with retention times 8.22, 8.68, 9.25, 11.36, 12.83 and 14.68 in watermelon plant, respectively [Table 3; Figures 1a–6].
Table 3.
Non-fatty acids compounds composition of Citrullus vulgaris (watermelon) and Cucumis melo (Muskmelon) detected by gas chromatography/mass spectroscopy
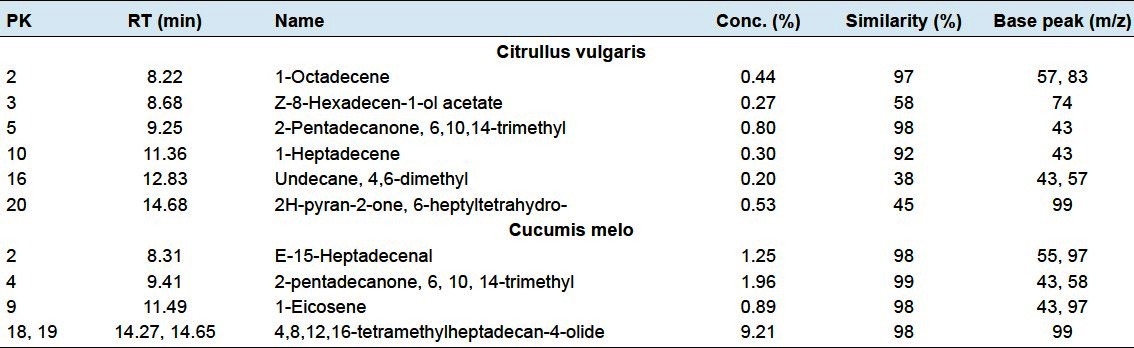
Figure 6.
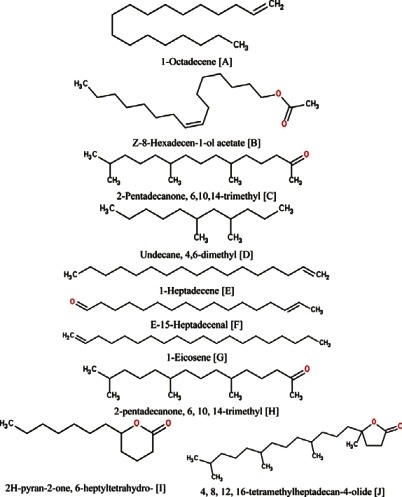
Ten non-fatty acids found in the two plants Citrullus vulgaris (watermelon) and Cucumis melo (Muskmelon)
The muskmelon plant contained 6 saturated fatty acids called tetradecanoic acid, pentadecanoic acid, hexadecanoic acid, heptadecanoic acid, eicosanoic acid and octadecanoic acid at 7.37, 9.00, 10.33, 12.19, 12.60 and 13.91 min retention time, respectively [Table 1; Figures 1b and 4]. The most abundant one is hexadecanoic acid with 39.36%, followed by octadecanoic acid with 10.45% [Figure 3], while the rest of fatty acids were minor. The saturated fatty acid derivatives of musckmelon plant were 5,9,13-trimethyl tetradecanoic acid, 14-methyl pentadecanoic acid, methyl-2,4-dimethyltetradecanoic acid, 10-methyl nonadecanoic acid and 3-hydroxy hexadecanoic acid at 9.75, 9.98, 11.79, 12.40 and 13.18 min. the most dominant one was 3-hydroxy-hexadecanoic acid with 5.72% [Table 1, Figure 4].
Figure 3.
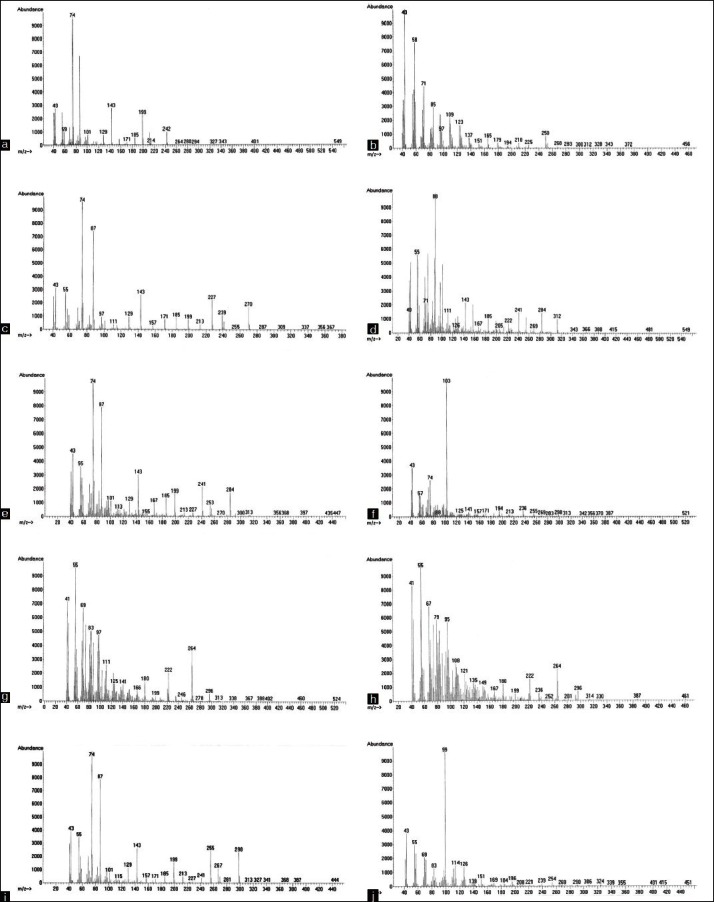
Mass spectra of the most abundant fatty acids in extracts of Cucumis melo (Muskmelon) plant. (a) Tetradecanoic acid, (b) 6,10,14-trimethyl- 2-Pentadecanone, (c) Hexadecanoic acid, (d) Methyl-2,4-dimethyl-Tetradecanoate, (e) Heptadecanoic acid, (f) 3-hydroxy-Hexadecanoic acid, (g) 9-Octadecenoic acid, (h) 11-Octadecenoic acid, (i) Octadecanoic acid and (j) 4,8,12,16-tetramethyl-Heptadecan-4-olide
There are three unsaturated fatty acid in muskmelon plant named 9-hexadecenoic acid, 9-octadecenoic acid (Z) and 11-octadecanoic acid at 10.23, 13.44 and 13.70 min, respectively. The most abundant one is 9-octadecenoic acid (Z) with 10.12% [Table 2; Figures 3 and 5].
The muskmelon plant contained four non-fatty acid compounds 15-heptadecenal (E), 6,10,14-trimethyl-2-pentadecanone, 1-eicosene and 4,8,12,16-tetramethyl-heptadecan-4-olide at 8.91, 9.41, 11.49 and 14.27 min, respectively. The most dominant one is 4, 8, 12, 16-tetramethyl-geptadecan-4-olide with 9.21% [Table 3; Figure 6].
DISCUSSION
Fatty Acids are aliphatic carboxylic acid with varying hydrocarbon lengths at one end of the chain joined to terminal carboxyl (-COOH) group at the other end. The general formula is R-(CH2)n-COOH. Fatty acids are predominantly unbranched and those with even numbers of carbon atoms between 12 and 22 carbons long react with glycerol to form lipids (fat-soluble components of living cells) in plants, animals, and microorganisms. The terminal carbon atom is called the omega carbon atom. The term “omega-3 or omega-6” signifies that their single solid bond is occurred at carbon number 3 or 6 respectively counted from and including the omega carbon. Human bodies are not capable of synthesizing omega-3 and omega-6 fatty acids which are called essential fatty acids must be obtained through the diet. These fatty acids were designated as “Vitamin F”, until it was realized that they must be classified with the fats. Fatty acids are converted to energy through the process called fatty acid oxidation in liver cells. Fatty acids are used as basic building blocks of biological membranes, for long-term energy storage (the major components of triglycerides) as well as for the precursors of eicosanoid hormones.[9–12]
In this study, both two plants (watermelon and muskmelon) contained five saturated fatty acids named tetrdecanoic acid, pentadecanoic acid, hexadecanoic acid, heptadecanoic acid and octadecanoic acid with different concentrations, while muskmelon contains an extra saturated fatty acid named eicosanoic acid. The watermelon plant contains five unsaturated fatty acids while muskmelon contains three only, the two plants share in two unsaturated fatty acids named 9-hexadecenoic acid and 9-octadecenoic acid, the muskmelon plant contains higher amounts of these two acids (2.04% and 10.12%, respectively) over watermelon plant (0.88% and 0.25%, respectively).
Composition of glyceridic fraction of four cucurbitaceae seed species from congo brazzaville was studied in order to evaluate intra et inter specific variability. This is a pre-condition to any selection work because seeds with lower variability lead to constant quality oils. It shows that two species (Cucurbita moschata (cm) and Citrullus lanatus (cl)) have lowest intra specific variability, considering all of fatty acids, fa (cv < 20%). their seeds lead to homogenous population. If one takes in account linoleic acid (c18:2), the most important essential fatty acid of edible oils, this variability falls respectively to 20 and 10%; consequently the seeds of the four species (Cucurbita moschata, Citrullus lanatus (cl), Cucurbita pepo (cp) and Lagenaria siceraria (ls)) present the same nutritional interest. Inter specific variability which is higher than intra one, leads to a good separation of the species. Multivariate analysis (analysis in principal components (acp), ascending hierarchical clustering (ahc) confirms this results and highlights the homogeneity of each species seeds and the good discrimination of the different species.[13]
Some chemical properties of Cucurbitaceae seed oils from different areas in Cameroon. These seeds are Cucumeropsis mannii (egusi melon), Cucurbita maxima (pumpkin or squash gourd), Cucurbita moschata (musk melón), Lagenaria siceraria (bottle gourd or calabash) and Cucumis sativus (“Ibo”egusi). The values for the indices are within recommended levels for edible oils. These oils have four main fatty acids: Linoleic acid, C18:2 (49-69%); oleic acid, C18:1 (9-25%); stearic acid, C18:O (7-11%) and palmitic acid, C16:O (10- 19%).[14]
Winter melon (Benincasa hispida), locally known as Kundur, is a vegetable crop, popular, especially among Asian communities both for nutritional and medicinal attributes. According to the GLC analysis linoleic acid (C18:2) was established to be the principal fatty acid (63.10-70.64%) followed by C16:0 (12.45-17.59), C18:1 (8.46-12.87%) and C18:0 (5.13-7.48%). Analysis of oil sterol fractions, using GC and GC-MS, revealed the presence of β-sitosterol (54.62-60.50%), campesterol (15.10-18.50%), stigmasterol (11.00-14.30% and Delta(5)-avenasterol (6.40-8.14%) as the four main components. Most of the properties of the seed oils analyzed varied significantly among fruit cultivars tested.[15]
Physico-chemical characteristics and fatty acids composition of kakri (Cucumis momordica L.) seeds oil were determined. Oil contents, protein contents, moisture content, free fatty acid, saponification values, iodine values, refractive index, specific gravity, unsaponifiable matter and fatty acids composition of oils extracted from seeds were determined. The oil content was 22.22%. The oil consists of 68.16% of unsaturated fatty acids and the main fatty acids are linoleic acid (52.63%), palmitic acid (21.05%), oleic acid (14.33%), stearic acid (10.79%) and linolenic acid (1.20%).[16]
The seeds from Kundur [Benincasa hispida (Thunb.) Cogn.], a fruit vegetable plant with high functional properties (especially in medicinal treatment), were analysed for nutritive parameters (dietary fiber, crude protein, crude fat, crude fiber, ash, and energy) and oil fatty acids composition. The contents of crude fat and crude protein were found to be 20.70 and 11.63%, respectively. The extracted Kundur seed oil mainly consisted of linoleic acid (C18:2ω6), accounting for 67.37% of the total fatty acids. Other important fatty acid detected were palmitic (C16:0), oleic (C18:1 cis) and stearic (C18:0) acids with contribution of 17.11, 10.21 and 4.83%, respectively. The fatty acids profile of the tested Kundur seed oil was quite comparable to those of previously reported cucurbitaceae seed oils and some other wholesome oils, revealing that it can be used as a potential seed oil crop.[17]
Examination of lipid extracts from photodegraded senescent phytoplanktonic cells demonstrates that autoxidation of vitamin E operated in phytodetritus, affording 4,8,12,16-tetramethylheptadecan-4-olide. In vitro, autoxidation of vitamin E is a rapid process under environmental conditions.[18]
In order to evaluate the differences and similarities between the liposoluble constituents in Cynomorium songaricum populations, stem liposoluble constituents in five populations of C. songaricum collected from three different geographic regions and four different hosts were obtained by solvent extraction and analyzed by GC–MS. Cluster analysis of the percentage composition of 80 compounds showed differences in chemical composition which were related to the geographic origin rather than the host. Hexadecanoic acid was the most abundant compound in the essential oils of C. songaricum from hosts Nitraria sibirica and Nitraria tanguticum. Whereas (Z)-9-octadecenoic acid was accumulated in the oils of C. songaricum from Zygophyllum xanthoxylum and Peganum harmala. Four of the five populations had characteristic components, which were specific to each population.[19]
Sabra et al.[20] investigated that the characteristic phytochemical profile of caffeic acid derivatives, alkamides and/or ketones was not affected by salinity. However, significant changes in their relative amount were found depending on the species and salinity intensity. In contrast, in Echinacea pallida, caftaric acid, echinacoside, and major ketones 22, 24, 25 and pentadeca-8Z, 11Z-dien-2-one levels were reduced at 50 and/or 75 mM NaCl. The highest concentration of salt (100 mM NaCl) reduced the level of cynarin, cichoric acid, cichoric acid derivative and alkamides 1, 3, 6, 7, 8/9 in E. purpurea and caftaric acid, alkamide 2, ketones 24, 25 in E. pallida, as well as alkamides 1, 2, 11 in E. angustifolia.
Low oil accumulation was observed during the first 35 days after the fruiting (DAF) date (from 1.83% to 2.57%). After that, two phases were distinguished (35th until the 60th and 105th to the 145th DAF), where the rate of oil accumulation increased significantly. At the last stage of maturation, the lentisc fruits had the highest percentage of lipid content, 42.54%. The changing profile of fatty acids during maturation had been marked mainly by an increase in oleic acid content (from 19.49% to 50.72%) paralleling a decrease in linoleic acid content (from 42.5% to 21.75%). At the 15th DAF, the alpha-linolenic acid was found with a maximum of 13.81%. At full maturity, the main fatty acids were oleic acid, followed by palmitic and linoleic acid. Other fatty acids were present in trace proportions, such as palmitoleic, stearic, linolenic, gadoleic and arachidic acid. In all stages of ripening only four sterols were identified and quantified. β-Sitosterol was the major 4-desmethylsterol in samples tested, followed by campesterol. Cholesterol and stigmasterol were detected in trace amounts. During the first stage of ripening, the amount of total sterols was about 5.19/100 g of oil. It decreased to 0.43/100 g in the last stage. Sitosterol and campesterol showed nearly the same profile during the ripening of P. lentiscus fruit which could be linked to the relation between these compounds during their biosynthesis.[21]
The essential oil composition of Calendula arvensis was established for the first time using GC and GC/MS. Eighty-five essential oil components were identified, which accounted for 90.3 g/100 g of essential oil. The oil contained a high concentration of sesquiterpenes, of which δ-cadinene and α-cadinol were the main components. The chemical composition of 25 Corsican C. arvensis oils was analyzed to determine intraspecies variation in essential oil composition. A matrix linking essential oil composition to sample location was composed to identify relationships between concentrations of volatile samples and the geographical origins of samples. Two main groups of compounds were identified according to the amount of sesquiterpenic compounds (hydrocarbons and alcohols) and soil characteristics. Seasonal variation (winter vs. spring) in the concentrations of two major compounds during the flowering period was observed.[22]
An isotope labeling study in humans, it was concluded that the fraction of dietary stearic acid oxidatively desaturated to oleic acid was 2.4 times higher than the fraction of palmitic acid analogously converted to palmitoleic acid. Also, stearic acid was less likely to be incorporated into cholesterol esters. In epidemiologic and clinical studies stearic acid was associated with lowered LDL cholesterol in comparison with other saturated fatty acids.[23] These findings may indicate that stearic acid is healthier than other saturated fatty acids.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Mitei YC, Ngila JC, Yeboah SO, Wessjohann L, Schmidt J. NMR, GC-MS and ESI-FTICR-MS Profiling of Fatty Acids and Triacylglycerols in Some Botswana Seed Oils. J Am Oil Chem Soc. 2008;85:1021–32. [Google Scholar]
- 2.El-Adaway TA, Taha K. Characteristics and composition of watermelon, pumpkin, and paprika seed oils and flours. J Agric Food Chem. 2001a;49:1253–9. doi: 10.1021/jf001117+. [DOI] [PubMed] [Google Scholar]
- 3.El-Adaway TA, Taha K. Characteristics and composition of different seed oils and flours. Food Chem. 2001b;74:47–54. doi: 10.1021/jf001117+. [DOI] [PubMed] [Google Scholar]
- 4.De Melo MLS, Narain N, Bora PS. Characterization of some nutritional constituents of melon (Cucumis melo hybrid AF-522) seeds. Food Chem. 2000;68:411-. [Google Scholar]
- 5.Lazos ES. Certain functional properties of defatted pumpkin seed flour. J Food Sci. 1986;51:1382–3. doi: 10.1007/BF02193934. [DOI] [PubMed] [Google Scholar]
- 6.Nestel P, Clifton P, Noakes M. Effects of increasing dietary palmitoleic acid compared with palmitic and oleic acids on plasma lipids of hypercholesterolemic men. J Lipid Res. 1994;35:656–62. [PubMed] [Google Scholar]
- 7.Power GW, Cake MH, Newsholme EA. The influence of diet on the activity of carnitine palmitoyltransferase 1 toward a range of acyl CoA esters. Lipids. 1997;32:31–7. doi: 10.1007/s11745-997-0005-4. [DOI] [PubMed] [Google Scholar]
- 8.Morrison WR, Smith LM. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron trifluoride-methanol. J Lipid Res. 1964;5:600–8. [PubMed] [Google Scholar]
- 9.Gunstone FD, Padley FB. New York: Marcel Dekker, Inc; 1997. Lipid Technologies and Applications. [Google Scholar]
- 10.Gunstone FD, Harwood JL, Padley FB. 2nd ed. London, U.K: Chapman and Hall; 1994. The Lipid Handbook. [Google Scholar]
- 11.Whitney EN, Cataldo CB, Rolfes SR. 5th ed. Belmont, CA: West/Wadsworth; 1998. Understanding Normal and Clinical Nutrition; pp. 141–75. [Google Scholar]
- 12.Jones PJ, Papamandjaris AA. “Chapter 10- Lipids: Cellular Metabolism” IN Present Knowledge in Nutrition. In: Bowman BA, Russell RM, editors. 8th ed. Washington, DC: ILSI Press; 2001. pp. 104–14. [Google Scholar]
- 13.Silou T, Tsieri MM, Makany R, Tremolieres A, Heron S, Tchapla A. Composition and variability of glycerioic fraction of four species of edible cucurbitacee from congo brazzaville. Rivista Italiana Delle Sostanze Grass. 2008;85:229–38. [Google Scholar]
- 14.Fokou E, Achu MB, Kansci G, Ponka R, Fotso M, Tchiégang C, et al. Chemical Properties of Some Cucurbitaceae Oils from Cameroon. Pak J Nutr. 2009;8:1325–34. [Google Scholar]
- 15.Anwar F, Mohammad NA, Othman F, Saari N. Inter-varietal variation in the composition of seeds and seed oils from winter melon [benincasa hispida (thunb.) cogn.] fruit. Pak J Bot. 2011;43:2029–37. [Google Scholar]
- 16.Rathore S, Sherwani MR. Studies on physico-chemical properties and fatty acid composition of kakri (Cucumis momordica L.) seeds oil from the arid zone of Rajasthan. Asian J Chem. 2010;22:4261–4. [Google Scholar]
- 17.Sew CC, Zaini NA, Anwar F, Abdul Hamid A, Saari N. Nutritional composition and oil fatty acids of kundur [Benincasa hispida (Thunb.) Cogn.] seed. Pak J Bot. 2010;42:3247–55. [Google Scholar]
- 18.Rontani JF, Nassiry M, Mouzdahir A. Free radical oxidation (autoxidation) of α-tocopherol (vitamin E): A potential source of 4, 8, 12, 16-tetramethylheptadecan-4-olide in the environment. Organic Geochem. 2007;38:37–47. [Google Scholar]
- 19.Zhou YB, Ye RR, Lu XF, Lin PC, Yang SB, Yue PP, et al. GC–MS analysis of liposoluble constituents from the stems of Cynomorium songaricum. J Pharma Biomed Anal. 2009;49:1097–100. doi: 10.1016/j.jpba.2009.01.038. [DOI] [PubMed] [Google Scholar]
- 20.Sabra A, Adam L, Daayf F, Renault S. Salinity-induced changes in caffeic acid derivatives, alkamides and ketones in three Echinacea species. Environ Exp Bot. 2012;77:234–41. [Google Scholar]
- 21.Trabelsi H, Cherif OA, Sakouhi F, Villeneuve P, Renaud J, Barouh N, et al. Total lipid content, fatty acids and 4-desmethylsterols accumulation in developing fruit of Pistacia lentiscus L. growing wild in Tunisia. Food Chem. 2012;131:434–40. [Google Scholar]
- 22.Paolini J, Barboni T, Desjobert JM, Djabou N, Muselli A, Costa J. Chemical composition, intraspecies variation and seasonal variation in essential oils of Calendula arvensis L. Biochem. Systematics Ecol. 2010;38:865–74. [Google Scholar]
- 23.Hunter JE, Zhang J, Kris-Etherton PM. Cardiovascular disease risk of dietary stearic acid compared with trans, other saturated, and unsaturated fatty acids: A systematic review. Am J Clin Nutr. 2010;91:46–63. doi: 10.3945/ajcn.2009.27661. [DOI] [PubMed] [Google Scholar]


