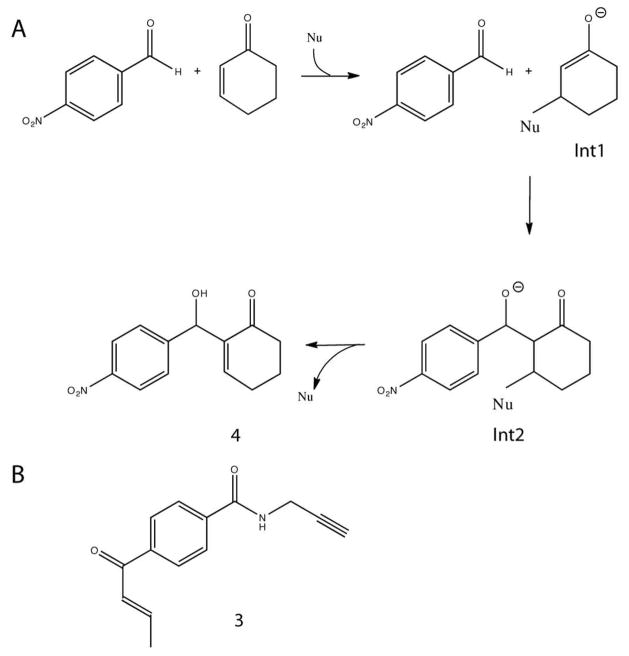
Figure 1.
(A) Morita-Baylis-Hillman reaction between cyclohexenone and 4-nitrobenzaldehyde proceeds via two intermediates Int1 and Int2. Int1 is enolate formed after the nucleophilic (Nu) attack on the C-beta carbon of the enone. Next 4-nitrobenzaldehyde attacks the enolate of Int1 to form Int2. The final product is released after the proton rearrangement and the catalyst is recycled. (B) Enone probe 3 (E)-(4-But-2-enoyl-N-(prop-2-ynyl)benzamide and MBH product 4 2-(Hydroxy(4-nitrophenyl)methyl)cyclohex-2-enone.