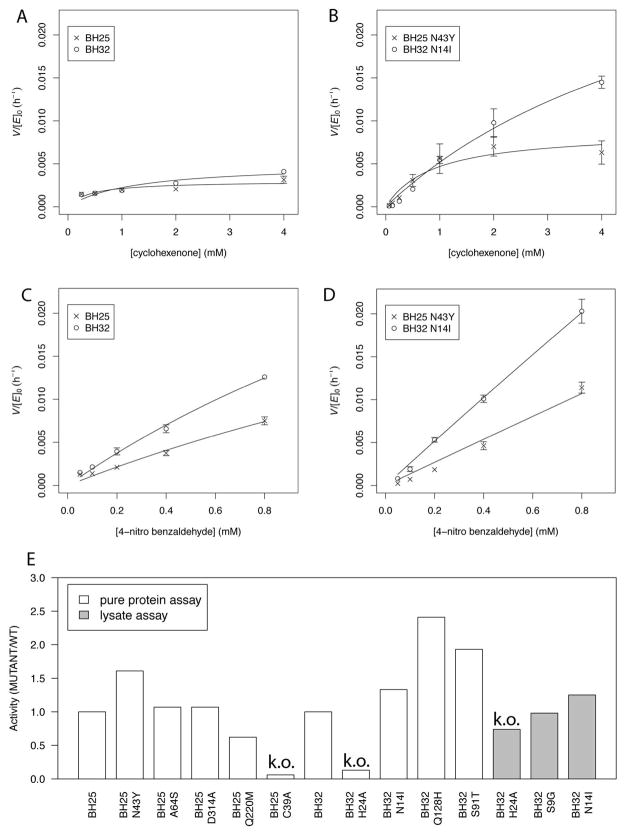
Figure 3.
Kinetic characterization of BH25, BH25 N43Y, BH32 and BH32 N14I variants and BH32 lysate assay. (A) BH25 and BH32, and (B) BH25 N43Y and BH32 N14I kinetics for fixed 400 μM concentration of 4-nitrobenzaldehyde with 0.25, 0.5, 1, 2 and 4 mM of cyclohexenone, respectively. The trend is similar for BH25 N43Y while no clear saturation is observed for BH32 and the BH32 N14I variant. (C) and (D) show kinetics for same MBH variants but with a fixed concentration of cyclohexenone at 4 mM while the 4-nitrobenzaldehyde concentration is fixed at 25, 50, 100, 200, 400 and 800 μM, respectively. The low solubility of 4-nitrobenzaldehyde prevents measurements at higher concentrations and no saturation is observed at 800 μM. (E) Pure protein and crude lysate reaction assay of MBH variants. C39A diminishes the BH25 activity about 20 times. BH32 Q128H mutant is the most active variant. BH32 N14I is detectable in lysate and the knockout mutant BH32 H24A is at the lysate background activity. Activities are normalized to BH25 and BH32 activity in both assays.