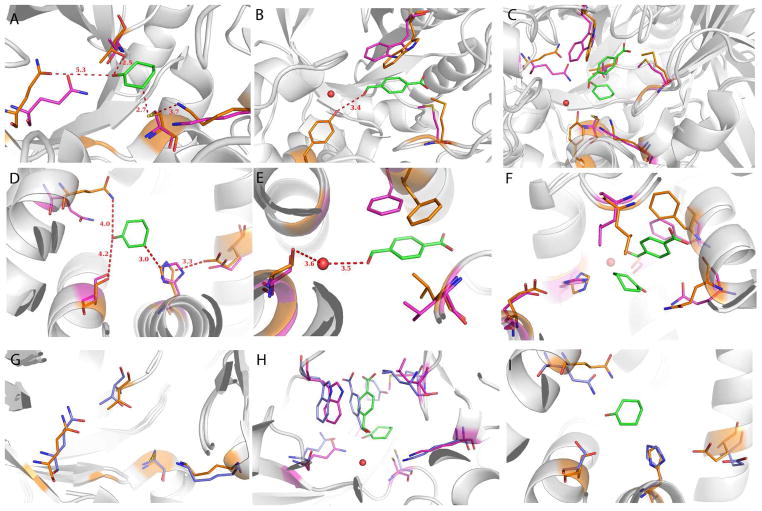
Figure 4.
Superposition of Morita-Baylis-Hillman (MBH) designs BH25 N43Y and BH32 (purple) on the corresponding X-ray structures, 3UW6 and 3U26 (orange). Both are apo structures, but for modeling purposes we superimpose the designed transition state model. (A) Cyclohexenone is positioned well for the nucleophilic attack by BH25 cysteine, (B) while the 4-nitrobenzaldehyde pocket shows some slight deviations from the design. A water molecule is depicted as a red sphere, which is occupied by N43Y mutant. (C) Transition state of the reaction as modeled during the design. (D) BH32 design shows similar binding of the cyclohexenone as BH25 design but with H23 as a nucleophile. Hydrogen-bonding Q128 adopts a different conformation from the designed one as Q219 in BH25 N42Y structure. (E) BH32 4-nitrobenzaldehyde binding pocket rearranges in the similar way as in BH25 N43Y. (F) BH32 with the transition state of the reaction depicting the whole active site. (G) The active site of BH25 after 50ns of MD (blue) superimposed on the crystal structure (orange). Note the good overlay of sidechains, indicating that the MD has correctly predicted both well-designed sidechains (C39 and K285) as well as those that shift from the design (Q219). (H) The active site of BH25 with cyclohexenone and 4-nitrobenzaldehyde substrates after 50 ns (blue) superimposed on the design structure (purple). (I) The active site of BH32 after 50 ns of MD (blue) superimposed on the crystal structure (orange).