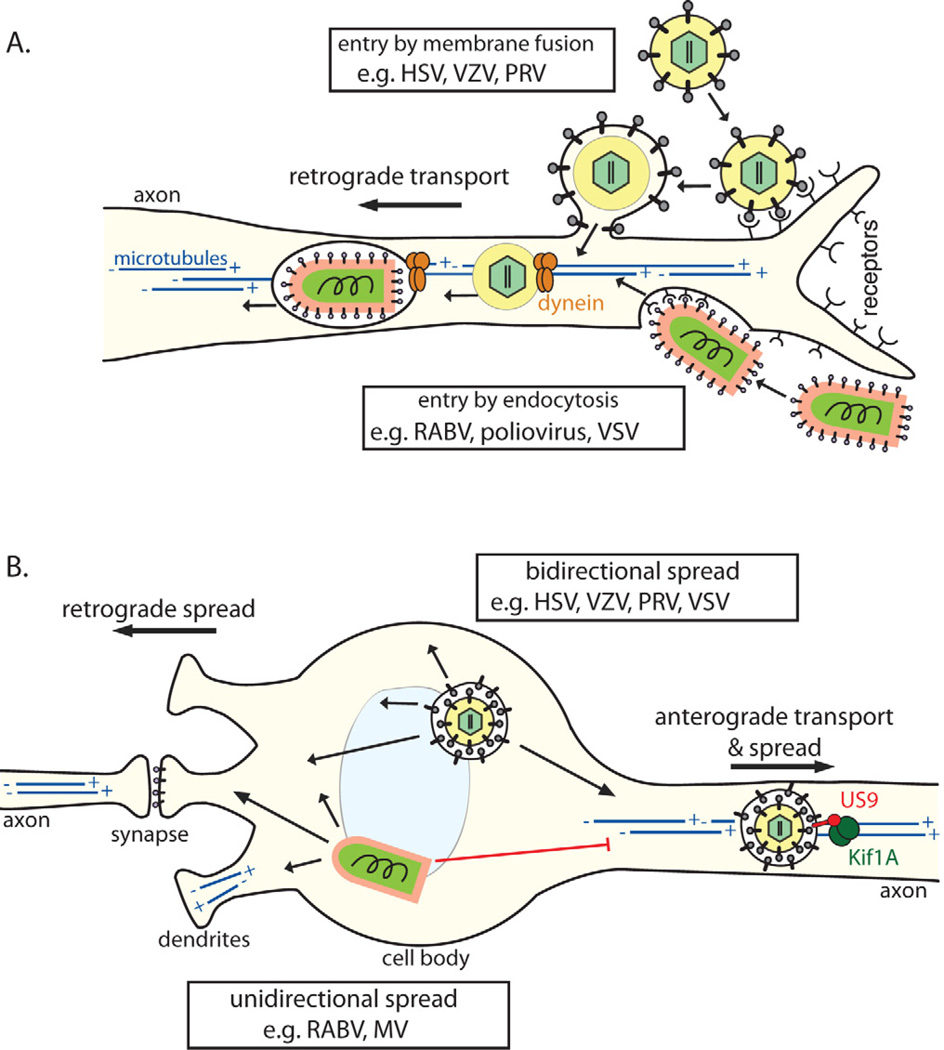
Figure 3. Cell biology of virus entry, transport, and spread in the NS.
(A) Virons enter axons either via (1) direct fusion with the axonal membrane after receptor attachment (e.g. alpha herpesviruses) or by (2) endocytosis (e.g. RABV; most neurotropic viruses). All particles entering the axon cytoplasm require (−) end-directed dynein motors for long distance retrograde transport on microtubules toward the cell body. Microtubules in axons are uniformly oriented with (+) ends facing the axon terminus and the (−) ends in the cell body. In the cell body and dendrites, microtubules have mixed polarity. (B) Post-replication trafficking and spread of progeny virions. Progeny alpha herpesvirus virions in transport vesicles can be transported anterograde in axons, dependent on the viral protein Us9 associating with the microtubule motor kinesin-3/Kif1A. Egress from axons allows anterograde virus spread from pre-synaptic to post-synaptic neurons. Virions in transport vesicles can also traffic to and egress from the somatodendritic compartment, allowing retrograde spread from post-synaptic to presynaptic neurons. Accordingly, herpesviruses can spread bidirectionally (1) in neuronal circuits. In contrast, enveloped RNA virions (e.g. RABV, MV) acquire their membranes by budding though the plasma membrane during egress. For these viruses, the location of envelopment/egress, and directionality of spread, may depend on the transport and intracellular localization of viral glycoproteins. These viruses tend to spread unidirectionally (2), only from post-synaptic to pre-synaptic neurons.