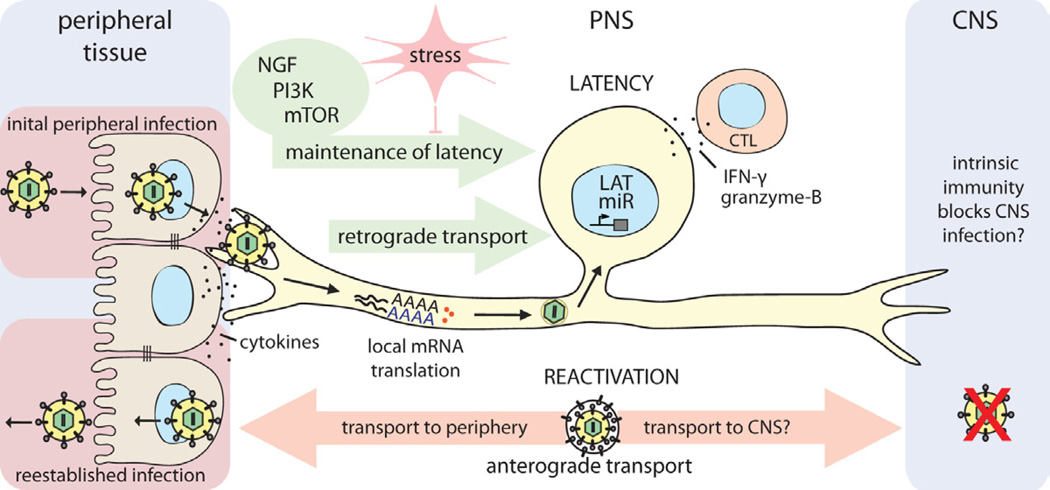
Figure 4. Establishment and control of alpha herpesvirus latency in PNS neurons.
HSV-1 enters the host organism through mucosal/epithelial surfaces. Initial replication in epithelial cells results both in cytokine secretion and viral spread. Progeny virions enter sensory nerve terminals and undergo long-distance retrograde transport to the neuronal cell body. Localized intracellular signaling and protein translation in distal axons induced by cytokines and viral proteins during entry supports retrograde particle transport and also may influence the establishment of latency in neuronal cell bodies. Once in the nucleus, viral latency genes are activated (e.g. LAT, miRs), and the HSV-1 genome is repressed. CD8+ CTLs secreting IFN-γ and granzyme-B, and continuous retrograde NGF-PI3K-mTOR signaling from distal axons contribute to maintaining this latent state of infection. If this homeostasis is disrupted by environmental or nutritional stress, the HSV-1 genome reactivates lytic gene expression, leading to virus replication. Newly replicated virions traffic back by anterograde axonal transport mechanisms, to reestablish infection at epithelial tissues. It is still not understood why CNS invasion is rare after reactivation from latency, even though both axonal projections of the pseudounipolar sensory neuron are accessible to HSV-1 virion transport. If there is no difference in transport, it may be that HSV-1 does invade the CNS, but is strongly suppressed or silenced by an effective intrinsic immune response in CNS neurons of healthy individuals.