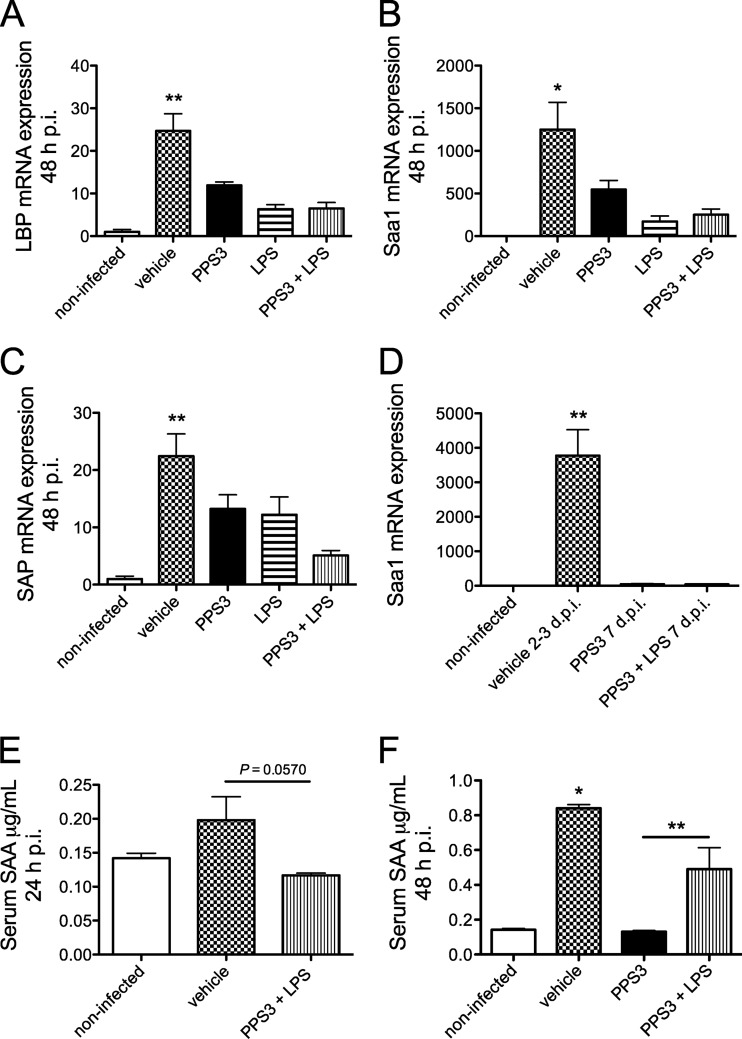
Fig 6.
Hepatic acute-phase protein mRNA and Saa protein expression levels in serum during the acute and resolution phases of infection with S. pneumoniae ST 3. Mice were immunized with vehicle (PBS) (n = 8), PPS3 (n = 8), LPS (n = 4), and PPS3+LPS (n = 8) 5 days prior to inoculation with 1 × 105 CFU (A to C) or 5 × 104 CFU (D) of S. pneumoniae ST 3. Liver mRNA expression was assessed at 48 h p.i. (A to C) and 7 days p.i. (D) by Q-PCR, and results were analyzed by the ΔΔCT method. Expression levels are shown as fold change in comparison to baseline noninfected values for LBP (A), Saa1 (B), and SAP (C) at 2 days p.i. and for Saa1 at 7 days p.i. (D). An ELISA examining serum SAA expression was conducted on blood samples collected via the vena cava at 24 h p.i. after inoculation with 5 × 104 CFU (E) and at 48 h p.i. after inoculation with 1 × 105 CFU (F) of S. pneumoniae ST 3. A Student's t test and one-way ANOVA were used for statistical analysis at 24 and 48 h p.i., respectively. Bars denote means plus standard errors of the means; asterisks denote P values of <0.05 (*) and <0.01 (**), as determined by one-way ANOVA with Tukey's posthoc test in comparison to infected control values.