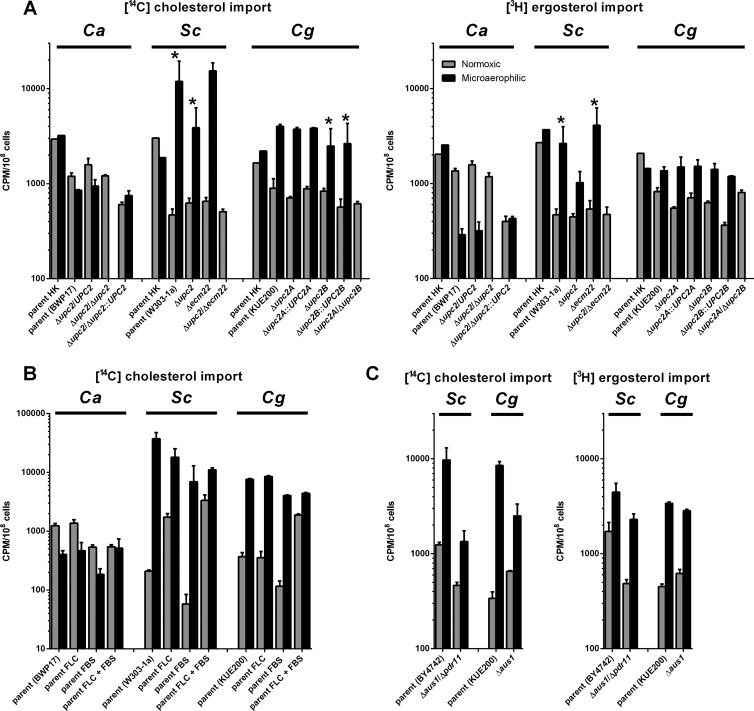
Fig 1.
Sterol uptake in C. albicans, S. cerevisiae, and C. glabrata strains under various conditions. Cholesterol (A, B, and C) and ergosterol (A and C) uptake under normoxic and microaerophilic conditions into the strains of three fungal species. Sterol import was measured in parental strains and their UPC2-deficient derivatives (A), parental strains in the presence of fluconazole (FLC) and/or fetal bovine serum (FBS) (B), or in parental strains and their AUS1- and PDR11-deficient derivatives (C), comparing heat-killed (HK) cells with actively growing strains. The graphs in panel C represent separate experiment from those in panel A and thus are presented separately. In all cases strains were grown for 48 h under normoxic (gray bars) and microaerophilic condition (black bars) in CSM complete supplemented with uridine, oleic acid in the form of Tween 80, and the appropriate sterol (cholesterol or ergosterol) with its radiolabeled form. In the experiment in panel C, fluconazole (FLC; each strain's MIC80) and/or fetal bovine serum (FBS; 10%) was added as noted. Samples were normalized to cpm/108 cells. Graphs represent the average of three biological replicates with the standard errors. All differences between normoxic and microaerophilic conditions are statistically significant as determined by using the Student t test (P < 0.05), and data pairs marked with asterisks are also significant (to P < 0.1). In panel B the difference between C. albicans cholesterol uptake in the presence of FLC and FBS is not statistically significant. Heat-killed controls were only performed once due to the number of samples in the experiment. The heat killing causes significant disruption of the cell membranes, as proven by an increase in propidium iodide permeability (45).