Abstract
The asymmetric unit of the title complex, [Fe2Ni(C19H12N3O)2(CN)6(C16H36N4)]·2.07H2O, contains one [Fe(qcq)(CN)3]− anion, half a [Ni(teta)]2+ cation and two partially occupied interstitial water molecules [qcq− is the N-(quinolin-8-yl)quinoline-2-carboxamidate anion and teta is 5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane]. In the complex molecule, two [Fe(qcq)(CN)3]− anions additionally coordinate the central [Ni(teta)]2+ cation through cyanide groups in a trans mode, resulting in a trinuclear structure with the Ni2+ cation lying on an inversion centre. The two interstitial water molecules are partially occupied, with occupancy factors of 0.528 (10) and 0.506 (9). O—H⋯O and O—H⋯N hydrogen bonding involving the two lattice water molecules and the carbonyl function and a teta N atom in an adjacent cluster leads to the formation of layers extending parallel to (010).
Related literature
For the synthesis and background to low-dimensional systems based on modified hexacyanidometalates, see: Liu et al. (2010 ▶); Kim et al. (2009 ▶); Curtis et al. (1964 ▶). For related structures, see: Li et al. (2012 ▶); Panja et al. (2012 ▶).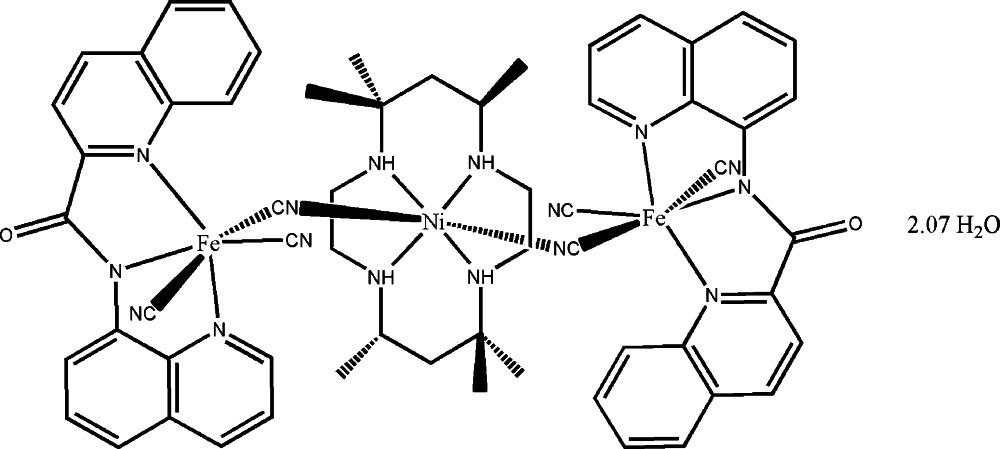
Experimental
Crystal data
[Fe2Ni(C19H12N3O)2(CN)6(C16H36N4)]·2.07H2O
M r = 1244.89
Monoclinic,
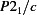
a = 9.4145 (13) Å
b = 15.7309 (17) Å
c = 20.590 (2) Å
β = 101.781 (3)°
V = 2985.1 (6) Å3
Z = 2
Mo Kα radiation
μ = 0.85 mm−1
T = 291 K
0.28 × 0.24 × 0.22 mm
Data collection
Rigaku Saturn 724 CCD diffractometer
Absorption correction: multi-scan (ABSCOR; Higashi, 1995 ▶) T min = 0.796, T max = 0.835
12764 measured reflections
5722 independent reflections
4078 reflections with I > 2σ(I)
R int = 0.022
Refinement
R[F 2 > 2σ(F 2)] = 0.057
wR(F 2) = 0.156
S = 0.97
5722 reflections
402 parameters
7 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.42 e Å−3
Δρmin = −0.42 e Å−3
Data collection: CrystalClear (Rigaku, 2008 ▶); cell refinement: CrystalClear; data reduction: CrystalClear; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXS97 (Sheldrick, 2008 ▶); molecular graphics: DIAMOND (Brandenburg, 2006 ▶); software used to prepare material for publication: SHELXTL (Sheldrick, 2008 ▶).
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536813010234/zl2544sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813010234/zl2544Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O2W—H2WA⋯O1 | 0.82 (2) | 2.14 (2) | 2.882 (5) | 151 (5) |
| O1W—H1WA⋯O2W | 0.85 (2) | 1.87 (7) | 2.623 (8) | 147 (11) |
| N8—H8A⋯O1W i | 0.91 | 2.19 | 3.091 (7) | 169 |
Symmetry code: (i) 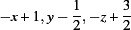 .
.
Acknowledgments
The authors thank the National Natural Science Foundation of China (No. 51072071) for financial support.
supplementary crystallographic information
Comment
Modified hexacyanometalates, [Fe(qcq)(CN)3]- (qcq- = 8-(2-quinoline-2-carboxamido)quinoline anion) have been shown to be effective building blocks that can be used instead of hexacyanometalates for the design of low dimensional assemblies (Liu et al., 2010). The capping ligand qcq- (Li et al., 2012) allows to limit oligomerization or polymerization effects by partially blocking the coordination sites around hexacyanometalates, and promotes the formation of low-dimensional structures. More importantly, it plays a crucial role in reducing the molecular symmetry, enhancing the anisotropy, and tuning the electronic, steric demand and solubility properties of derived complexes (Panja et al., 2012). However, to the best of our knowledge, low dimensional compounds based on [Fe(qcq)(CN)3]- as a ligand have been rarely explored and only a few related complexes have been reported so far. Therefore, the investigation of related low dimensional assemblies based on [Fe(qcq)(CN)3]- is of significance. Considering that the macrocyclic cation of [Ni(teta)]2+ (teta = 5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane) can behave as a good electron acceptor, our synthesis strategy is to employ [Fe(qcq)(CN)3]- and [Ni(teta)]2+ as precursors to construct low dimensional assemblies. Herein, the crystal structure of a new trinuclear complex, [{Ni(teta)}{Fe(qcq)(CN)3}2].2H2O is presented.
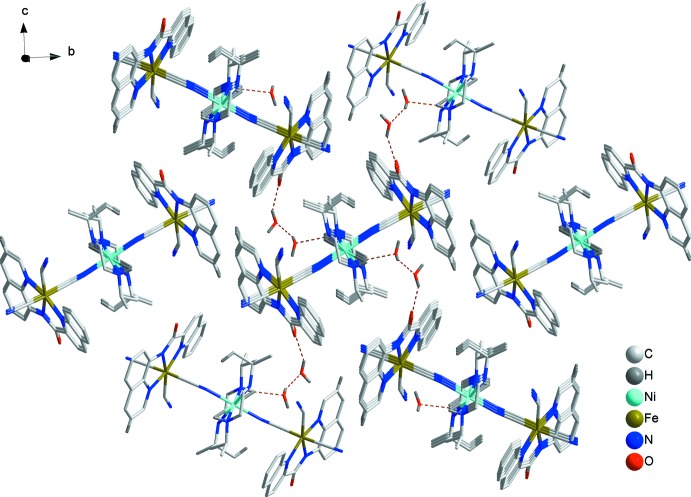
The molecular structure of the title complex is shown in Fig. 1. Within the neutral trinuclear clusters, two [Fe(qcq)(CN)3]- anions coordinate to the central [Ni(teta)]2+ cation in a trans-mode, resulting in a nearly linear and centrosymmetric structure, where the Ni atom lies on an inversion centre. For the moieties of [Fe(qcq)(CN)3]-, the central Fe ion is coordinated by three C atoms from cyanide groups (Fe—C(cyanide) bond lengths: 1.951 (4)–1.965 (4) Å) and three N atoms from qcq- (Fe—N(qcq) bond lengths: (1.970 (4)–2.146 (3) Å), affording a distorted octahedral coordination for the metal centre. The Fe—N (amide) bond length (1.970 (4) Å) is shorter than those for the Fe—N (aromatic rings) (2.045 (4)–2.146 (3) Å), which can be attributed to the strong σ-donor effect of the deprotonated amide. The bond angles of Fe1—C1—N1 and Fe1—C2—N3 remain almost linear (172.6 (3)–179.1 (4)°), while the Fe1—C3—N2 one deviates significantly from linearity (150.7 (5) °). The bond angle of Ni—N—C(cyanide) also deviates from linearity (161.1 (3)°), which is comparable to values observed in many other cyano-bridged bimetallic assemblies (Kim et al., 2009). For the structural unit of [Ni(teta)]2+, the equatorial sites of the central Ni ion are occupied by four nitrogen atoms from the macrocyclic ligand of teta (Ni—Nmacro bond lengths: 2.077 (3)–2.092 (3) Å), while the axial positions are occupied by Ncyanide from [Fe(qcq)(CN)3]- (Ni—Ncyanide bond lengths: 2.116 (3) Å). The intramolecular Fe···Ni distance is 5.101 (3) Å. For the intermolecular interactions, the interstitial water molecules are positioned between the clusters and linked to the nitrogen atom of teta and the oxygen atom of adjacent clusters via hydrogen bonds, further extending the dimensionality of the structure to a supramolecular network, as shown in Fig. 2.
Experimental
The complex was obtained as black block crystals by slow diffusion of a methanol solution (5 ml) of PPh4[Fe(qcq)(CN)3] (0.10 mmol) (Kim et al., 2009) and a water/DMF (v:v = 7:8) solution (15 ml) of [Ni(teta)](ClO4)2 (0.10 mmol) (Curtis et al., 1964) through a H-shaped tube at room temperature for about two weeks. The resulting crystals were collected, washed with H2O and CH3OH, respectively, and dried in air. Anal. found: C, 57.70; H, 5.23; N, 18.04; Fe, 8.92; Ni, 4.87%. Calcd for C60H64.14Fe2N16NiO4.07: C, 57.95; H, 5.19; N, 18.02; Fe, 8.98; Ni, 4.72%.
Refinement
All non-H atoms were refined with anisotropic thermal parameters. The C– and N-bound H atoms were placed in idealized positions and included in the refinement in a riding mode (C—H = 0.95 Å, N—H = 0.88 Å) with Uiso for H assigned as 1.2 or 1.5 times Ueq of the attached atoms. The oxygen atoms (O1W, O2W) of interstitial water molecules are refined with partial occupancy factors of 0.528 (10) for the water molecule of O1W and 0.506 (9) for that of O2W, respectively. The water H-atoms were located from difference maps and were refined with a O—H and H···H distance restraints of 0.82 (2) Å) and 1.36 (2) Å and with Uiso for H assigned as 1.5 times Ueq of the attached atoms. The H atom H2WA was further restrained to be 2.10 (2) Å from O1 to rationalize the hydrogen bonds interactions.
Figures
Fig. 1.
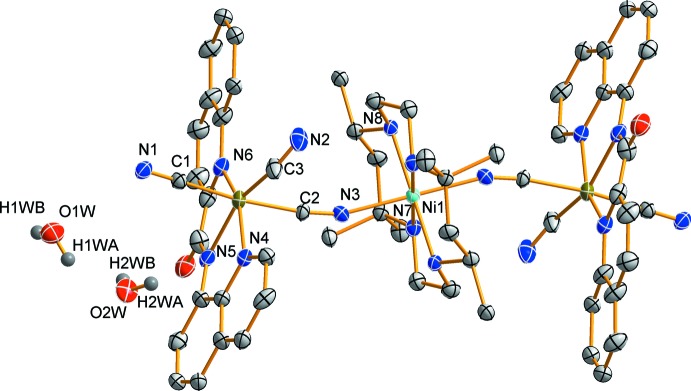
ORTEP diagram of the title complex with displacement ellipsoids drawn at the 30% probability level (The non-solvent H atoms have been omitted for clarity).
Fig. 2.
The packing and intermolecular interactions for the title complex (The dotted line represents the N—H···O and O—H···O hydrogen bonds).
Crystal data
| [Fe2Ni(C19H12N3O)2(CN)6(C16H36N4)]·2.07H2O | F(000) = 1297.4 |
| Mr = 1244.89 | Dx = 1.384 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 3735 reflections |
| a = 9.4145 (13) Å | θ = 2.1–23.4° |
| b = 15.7309 (17) Å | µ = 0.85 mm−1 |
| c = 20.590 (2) Å | T = 291 K |
| β = 101.781 (3)° | Block, black |
| V = 2985.1 (6) Å3 | 0.28 × 0.24 × 0.22 mm |
| Z = 2 |
Data collection
| Rigaku Saturn 724 CCD diffractometer | 5722 independent reflections |
| Radiation source: fine-focus sealed tube | 4078 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.022 |
| φ and ω scans | θmax = 26.0°, θmin = 3.3° |
| Absorption correction: multi-scan (ABSCOR; Higashi, 1995) | h = −11→11 |
| Tmin = 0.796, Tmax = 0.835 | k = 0→19 |
| 12764 measured reflections | l = 0→25 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.057 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.156 | H atoms treated by a mixture of independent and constrained refinement |
| S = 0.97 | w = 1/[σ2(Fo2) + (0.095P)2] where P = (Fo2 + 2Fc2)/3 |
| 5722 reflections | (Δ/σ)max < 0.001 |
| 402 parameters | Δρmax = 0.42 e Å−3 |
| 7 restraints | Δρmin = −0.42 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. the restraints that were used for the refinement of the water H atoms are: DFIX 2.1 H2WA O1 DFIX 1.36 H1WA H1WB H2WA H2WB DFIX 0.82 O1W H1WA O1W H1WB O2W H2WA O2W H2WB |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| C1 | 0.6080 (3) | 0.3867 (2) | 0.85132 (16) | 0.0385 (8) | |
| C2 | 0.4738 (3) | 0.1784 (2) | 0.92238 (15) | 0.0381 (8) | |
| C3 | 0.6764 (4) | 0.2943 (3) | 0.9657 (2) | 0.0603 (12) | |
| C4 | 0.4671 (4) | 0.3845 (2) | 1.00163 (18) | 0.0439 (8) | |
| H4 | 0.5580 | 0.3678 | 1.0250 | 0.053* | |
| C5 | 0.3788 (4) | 0.4394 (3) | 1.03091 (17) | 0.0475 (10) | |
| H5 | 0.4135 | 0.4595 | 1.0737 | 0.057* | |
| C6 | 0.2454 (4) | 0.4634 (3) | 0.99820 (18) | 0.0535 (10) | |
| H6 | 0.1895 | 0.4999 | 1.0182 | 0.064* | |
| C7 | 0.1919 (4) | 0.4330 (3) | 0.93380 (19) | 0.0507 (10) | |
| C8 | 0.0562 (4) | 0.4568 (3) | 0.89958 (18) | 0.0479 (9) | |
| H8 | −0.0011 | 0.4932 | 0.9188 | 0.057* | |
| C9 | 0.0051 (4) | 0.4247 (3) | 0.83428 (19) | 0.0556 (11) | |
| H9 | −0.0875 | 0.4387 | 0.8113 | 0.067* | |
| C10 | 0.0903 (4) | 0.3742 (3) | 0.80565 (18) | 0.0480 (9) | |
| H10 | 0.0570 | 0.3549 | 0.7626 | 0.058* | |
| C11 | 0.2293 (4) | 0.3504 (3) | 0.84038 (18) | 0.0492 (9) | |
| C12 | 0.2803 (3) | 0.3806 (3) | 0.90398 (17) | 0.0431 (8) | |
| C13 | 0.3175 (4) | 0.2439 (3) | 0.76782 (19) | 0.0531 (10) | |
| C14 | 0.4306 (4) | 0.2005 (3) | 0.76050 (17) | 0.0470 (9) | |
| C15 | 0.4349 (4) | 0.1491 (3) | 0.70543 (19) | 0.0535 (10) | |
| H15 | 0.3499 | 0.1343 | 0.6759 | 0.064* | |
| C16 | 0.5658 (4) | 0.1213 (3) | 0.6960 (2) | 0.0601 (11) | |
| H16 | 0.5689 | 0.0894 | 0.6583 | 0.072* | |
| C17 | 0.6919 (4) | 0.1378 (3) | 0.73891 (19) | 0.0549 (10) | |
| C18 | 0.8243 (4) | 0.1087 (3) | 0.7271 (2) | 0.0565 (11) | |
| H18 | 0.8272 | 0.0769 | 0.6893 | 0.068* | |
| C19 | 0.9535 (4) | 0.1276 (3) | 0.7727 (2) | 0.0536 (10) | |
| H19 | 1.0420 | 0.1080 | 0.7652 | 0.064* | |
| C20 | 0.9488 (4) | 0.1744 (3) | 0.8271 (2) | 0.0539 (10) | |
| H20 | 1.0342 | 0.1857 | 0.8576 | 0.065* | |
| C21 | 0.8156 (4) | 0.2063 (3) | 0.8384 (2) | 0.0534 (10) | |
| H21 | 0.8136 | 0.2406 | 0.8750 | 0.064* | |
| C22 | 0.6895 (4) | 0.1863 (3) | 0.79498 (18) | 0.0462 (9) | |
| C23 | 0.3339 (3) | −0.0867 (2) | 0.86776 (16) | 0.0393 (8) | |
| C24 | 0.4859 (4) | −0.1054 (3) | 0.85829 (17) | 0.0447 (9) | |
| H24A | 0.5204 | −0.1545 | 0.8855 | 0.054* | |
| H24B | 0.4782 | −0.1227 | 0.8125 | 0.054* | |
| C25 | 0.6028 (4) | −0.0390 (2) | 0.87275 (16) | 0.0393 (8) | |
| H25 | 0.5618 | 0.0160 | 0.8561 | 0.047* | |
| C26 | 0.7755 (4) | 0.0258 (3) | 0.96768 (17) | 0.0453 (9) | |
| H26A | 0.7525 | 0.0793 | 0.9444 | 0.054* | |
| H26B | 0.8632 | 0.0035 | 0.9562 | 0.054* | |
| C27 | 0.1984 (4) | −0.0414 (3) | 0.95781 (16) | 0.0449 (9) | |
| H27A | 0.1243 | −0.0845 | 0.9453 | 0.054* | |
| H27B | 0.1649 | 0.0106 | 0.9342 | 0.054* | |
| C28 | 0.2337 (4) | −0.1648 (3) | 0.84162 (17) | 0.0476 (9) | |
| H28A | 0.1373 | −0.1542 | 0.8483 | 0.071* | |
| H28B | 0.2716 | −0.2150 | 0.8655 | 0.071* | |
| H28C | 0.2312 | −0.1728 | 0.7952 | 0.071* | |
| C29 | 0.2665 (4) | −0.0076 (3) | 0.82503 (19) | 0.0472 (9) | |
| H29A | 0.2796 | 0.0425 | 0.8522 | 0.071* | |
| H29B | 0.1648 | −0.0171 | 0.8085 | 0.071* | |
| H29C | 0.3142 | −0.0003 | 0.7884 | 0.071* | |
| C30 | 0.7320 (4) | −0.0618 (3) | 0.83749 (18) | 0.0462 (9) | |
| H30A | 0.7018 | −0.1060 | 0.8054 | 0.069* | |
| H30B | 0.8137 | −0.0811 | 0.8700 | 0.069* | |
| H30C | 0.7589 | −0.0123 | 0.8156 | 0.069* | |
| Ni1 | 0.5000 | 0.0000 | 1.0000 | 0.0353 (2) | |
| Fe1 | 0.52931 (5) | 0.28363 (4) | 0.88333 (2) | 0.04223 (18) | |
| N1 | 0.6534 (3) | 0.4476 (2) | 0.83299 (14) | 0.0454 (7) | |
| N2 | 0.7764 (3) | 0.2701 (3) | 1.00090 (15) | 0.0622 (10) | |
| N3 | 0.4567 (3) | 0.1146 (2) | 0.94597 (14) | 0.0420 (7) | |
| N4 | 0.4158 (3) | 0.3561 (2) | 0.93779 (15) | 0.0461 (8) | |
| N5 | 0.3195 (3) | 0.2951 (2) | 0.81931 (14) | 0.0445 (7) | |
| N6 | 0.5568 (3) | 0.2176 (2) | 0.80568 (15) | 0.0478 (8) | |
| N7 | 0.3381 (3) | −0.0703 (2) | 0.93949 (14) | 0.0412 (7) | |
| H7 | 0.3509 | −0.1232 | 0.9576 | 0.049* | |
| N8 | 0.6599 (3) | −0.0325 (2) | 0.94730 (12) | 0.0363 (6) | |
| H8A | 0.6936 | −0.0848 | 0.9616 | 0.044* | |
| O1 | 0.2120 (3) | 0.2298 (2) | 0.72506 (14) | 0.0600 (8) | |
| O1W | 0.2133 (5) | 0.2873 (4) | 0.5217 (3) | 0.066 (2) | 0.528 (10) |
| H1WA | 0.142 (7) | 0.280 (7) | 0.540 (4) | 0.100* | 0.528 (10) |
| H1WB | 0.186 (9) | 0.273 (8) | 0.4823 (19) | 0.100* | 0.528 (10) |
| O2W | 0.0492 (5) | 0.2088 (4) | 0.5914 (2) | 0.059 (2) | 0.506 (9) |
| H2WA | 0.089 (9) | 0.196 (3) | 0.6296 (15) | 0.088* | 0.506 (9) |
| H2WB | 0.051 (11) | 0.177 (4) | 0.562 (2) | 0.088* | 0.506 (9) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0440 (16) | 0.032 (2) | 0.0422 (18) | 0.0046 (15) | 0.0141 (14) | 0.0035 (16) |
| C2 | 0.0486 (17) | 0.040 (2) | 0.0293 (16) | 0.0066 (15) | 0.0166 (13) | 0.0102 (15) |
| C3 | 0.0380 (17) | 0.065 (3) | 0.071 (3) | −0.0173 (17) | −0.0054 (17) | 0.033 (2) |
| C4 | 0.0407 (15) | 0.039 (2) | 0.053 (2) | 0.0031 (16) | 0.0106 (14) | 0.0069 (17) |
| C5 | 0.0520 (18) | 0.053 (3) | 0.0423 (18) | −0.0232 (17) | 0.0210 (15) | −0.0186 (18) |
| C6 | 0.064 (2) | 0.053 (3) | 0.050 (2) | 0.013 (2) | 0.0258 (18) | 0.015 (2) |
| C7 | 0.0521 (19) | 0.041 (2) | 0.063 (2) | 0.0119 (17) | 0.0223 (17) | 0.0165 (19) |
| C8 | 0.0507 (18) | 0.049 (3) | 0.049 (2) | 0.0148 (17) | 0.0218 (16) | 0.0165 (19) |
| C9 | 0.0426 (17) | 0.070 (3) | 0.057 (2) | 0.0203 (18) | 0.0152 (16) | 0.028 (2) |
| C10 | 0.0452 (17) | 0.051 (3) | 0.0476 (19) | −0.0102 (17) | 0.0088 (15) | −0.0018 (18) |
| C11 | 0.0509 (18) | 0.047 (3) | 0.053 (2) | 0.0161 (17) | 0.0185 (16) | 0.0152 (19) |
| C12 | 0.0443 (17) | 0.043 (2) | 0.0457 (19) | 0.0084 (16) | 0.0187 (15) | 0.0112 (17) |
| C13 | 0.066 (2) | 0.047 (3) | 0.043 (2) | −0.0011 (19) | 0.0043 (18) | −0.0028 (19) |
| C14 | 0.0513 (18) | 0.052 (3) | 0.0377 (18) | −0.0157 (17) | 0.0102 (14) | −0.0001 (17) |
| C15 | 0.0537 (19) | 0.054 (3) | 0.054 (2) | −0.0229 (18) | 0.0128 (16) | −0.017 (2) |
| C16 | 0.054 (2) | 0.060 (3) | 0.069 (3) | −0.002 (2) | 0.0193 (19) | −0.004 (2) |
| C17 | 0.058 (2) | 0.057 (3) | 0.056 (2) | −0.0156 (19) | 0.0268 (18) | −0.015 (2) |
| C18 | 0.062 (2) | 0.059 (3) | 0.056 (2) | −0.009 (2) | 0.0284 (19) | −0.018 (2) |
| C19 | 0.0438 (18) | 0.055 (3) | 0.065 (2) | 0.0123 (17) | 0.0201 (17) | 0.018 (2) |
| C20 | 0.0451 (17) | 0.055 (3) | 0.065 (2) | 0.0106 (17) | 0.0180 (17) | 0.023 (2) |
| C21 | 0.0485 (18) | 0.046 (3) | 0.072 (3) | 0.0142 (17) | 0.0268 (18) | 0.021 (2) |
| C22 | 0.0518 (19) | 0.043 (2) | 0.050 (2) | −0.0107 (17) | 0.0247 (16) | −0.0084 (18) |
| C23 | 0.0411 (15) | 0.035 (2) | 0.0445 (18) | 0.0026 (14) | 0.0162 (14) | 0.0054 (16) |
| C24 | 0.0525 (18) | 0.044 (2) | 0.0407 (18) | 0.0126 (17) | 0.0179 (15) | −0.0069 (17) |
| C25 | 0.0500 (17) | 0.036 (2) | 0.0348 (16) | 0.0095 (15) | 0.0165 (14) | 0.0013 (16) |
| C26 | 0.0458 (17) | 0.044 (2) | 0.050 (2) | 0.0035 (16) | 0.0183 (15) | −0.0004 (18) |
| C27 | 0.0433 (16) | 0.050 (3) | 0.0411 (18) | 0.0061 (16) | 0.0088 (14) | 0.0074 (18) |
| C28 | 0.0572 (19) | 0.040 (2) | 0.0416 (19) | −0.0095 (17) | 0.0016 (15) | −0.0048 (17) |
| C29 | 0.0477 (19) | 0.045 (3) | 0.046 (2) | −0.0012 (15) | 0.0013 (16) | 0.0163 (17) |
| C30 | 0.0503 (17) | 0.043 (2) | 0.049 (2) | 0.0150 (16) | 0.0192 (15) | 0.0126 (18) |
| Ni1 | 0.0407 (3) | 0.0359 (4) | 0.0322 (3) | 0.0059 (2) | 0.0141 (3) | 0.0083 (3) |
| Fe1 | 0.0481 (3) | 0.0385 (4) | 0.0415 (3) | −0.0006 (2) | 0.0123 (2) | 0.0128 (2) |
| N1 | 0.0450 (14) | 0.053 (2) | 0.0410 (15) | −0.0037 (14) | 0.0156 (12) | 0.0039 (15) |
| N2 | 0.0565 (18) | 0.075 (3) | 0.0459 (18) | −0.0148 (18) | −0.0120 (15) | 0.0167 (18) |
| N3 | 0.0428 (14) | 0.037 (2) | 0.0476 (17) | 0.0098 (13) | 0.0120 (12) | 0.0068 (15) |
| N4 | 0.0432 (13) | 0.049 (2) | 0.0480 (17) | −0.0025 (14) | 0.0137 (13) | 0.0156 (16) |
| N5 | 0.0503 (15) | 0.043 (2) | 0.0461 (16) | −0.0048 (14) | 0.0225 (13) | 0.0056 (14) |
| N6 | 0.0488 (15) | 0.045 (2) | 0.0516 (17) | −0.0068 (14) | 0.0159 (13) | 0.0114 (15) |
| N7 | 0.0451 (14) | 0.0350 (18) | 0.0457 (16) | 0.0004 (12) | 0.0143 (12) | 0.0065 (14) |
| N8 | 0.0425 (13) | 0.0369 (18) | 0.0326 (13) | 0.0078 (12) | 0.0145 (11) | 0.0066 (13) |
| O1 | 0.0569 (15) | 0.062 (2) | 0.0602 (16) | −0.0231 (14) | 0.0095 (13) | 0.0007 (15) |
| O1W | 0.052 (3) | 0.072 (5) | 0.074 (4) | −0.013 (3) | 0.009 (3) | −0.005 (3) |
| O2W | 0.054 (3) | 0.073 (5) | 0.039 (3) | −0.021 (3) | −0.017 (2) | −0.009 (3) |
Geometric parameters (Å, º)
| C1—N1 | 1.144 (5) | C22—N6 | 1.401 (5) |
| C1—Fe1 | 1.952 (4) | C23—N7 | 1.492 (4) |
| C2—N3 | 1.140 (5) | C23—C24 | 1.513 (4) |
| C2—Fe1 | 1.958 (4) | C23—C28 | 1.576 (5) |
| C3—N2 | 1.131 (5) | C23—C29 | 1.579 (5) |
| C3—Fe1 | 1.965 (4) | C24—C25 | 1.503 (5) |
| C4—N4 | 1.378 (5) | C24—H24A | 0.9700 |
| C4—C5 | 1.416 (5) | C24—H24B | 0.9700 |
| C4—H4 | 0.9300 | C25—N8 | 1.523 (4) |
| C5—C6 | 1.353 (5) | C25—C30 | 1.580 (4) |
| C5—H5 | 0.9300 | C25—H25 | 0.9800 |
| C6—C7 | 1.403 (6) | C26—N8 | 1.420 (5) |
| C6—H6 | 0.9300 | C26—C27i | 1.523 (5) |
| C7—C8 | 1.378 (5) | C26—H26A | 0.9700 |
| C7—C12 | 1.399 (5) | C26—H26B | 0.9700 |
| C8—C9 | 1.424 (6) | C27—N7 | 1.511 (4) |
| C8—H8 | 0.9300 | C27—C26i | 1.523 (5) |
| C9—C10 | 1.347 (6) | C27—H27A | 0.9700 |
| C9—H9 | 0.9300 | C27—H27B | 0.9700 |
| C10—C11 | 1.408 (5) | C28—H28A | 0.9600 |
| C10—H10 | 0.9300 | C28—H28B | 0.9600 |
| C11—N5 | 1.349 (5) | C28—H28C | 0.9600 |
| C11—C12 | 1.384 (5) | C29—H29A | 0.9600 |
| C12—N4 | 1.378 (4) | C29—H29B | 0.9600 |
| C13—O1 | 1.206 (5) | C29—H29C | 0.9600 |
| C13—C14 | 1.300 (6) | C30—H30A | 0.9600 |
| C13—N5 | 1.328 (5) | C30—H30B | 0.9600 |
| C14—N6 | 1.378 (5) | C30—H30C | 0.9600 |
| C14—C15 | 1.400 (5) | Ni1—N7i | 2.079 (3) |
| C15—C16 | 1.359 (5) | Ni1—N7 | 2.079 (3) |
| C15—H15 | 0.9300 | Ni1—N8i | 2.091 (2) |
| C16—C17 | 1.352 (6) | Ni1—N8 | 2.091 (2) |
| C16—H16 | 0.9300 | Ni1—N3i | 2.114 (3) |
| C17—C22 | 1.388 (5) | Ni1—N3 | 2.114 (3) |
| C17—C18 | 1.395 (5) | Fe1—N6 | 1.967 (3) |
| C18—C19 | 1.409 (5) | Fe1—N4 | 2.047 (3) |
| C18—H18 | 0.9300 | Fe1—N5 | 2.147 (3) |
| C19—C20 | 1.349 (6) | N7—H7 | 0.9100 |
| C19—H19 | 0.9300 | N8—H8A | 0.9100 |
| C20—C21 | 1.414 (5) | O1W—H1WA | 0.85 (2) |
| C20—H20 | 0.9300 | O1W—H1WB | 0.83 (2) |
| C21—C22 | 1.369 (5) | O2W—H2WA | 0.82 (2) |
| C21—H21 | 0.9300 | O2W—H2WB | 0.79 (2) |
| N1—C1—Fe1 | 179.3 (3) | N8—C26—H26B | 109.5 |
| N3—C2—Fe1 | 172.6 (3) | C27i—C26—H26B | 109.5 |
| N2—C3—Fe1 | 150.8 (5) | H26A—C26—H26B | 108.0 |
| N4—C4—C5 | 118.8 (3) | N7—C27—C26i | 109.3 (3) |
| N4—C4—H4 | 120.6 | N7—C27—H27A | 109.8 |
| C5—C4—H4 | 120.6 | C26i—C27—H27A | 109.8 |
| C6—C5—C4 | 121.6 (3) | N7—C27—H27B | 109.8 |
| C6—C5—H5 | 119.2 | C26i—C27—H27B | 109.8 |
| C4—C5—H5 | 119.2 | H27A—C27—H27B | 108.3 |
| C5—C6—C7 | 119.5 (4) | C23—C28—H28A | 109.5 |
| C5—C6—H6 | 120.2 | C23—C28—H28B | 109.5 |
| C7—C6—H6 | 120.2 | H28A—C28—H28B | 109.5 |
| C8—C7—C12 | 120.5 (4) | C23—C28—H28C | 109.5 |
| C8—C7—C6 | 120.4 (4) | H28A—C28—H28C | 109.5 |
| C12—C7—C6 | 119.0 (3) | H28B—C28—H28C | 109.5 |
| C7—C8—C9 | 119.1 (4) | C23—C29—H29A | 109.5 |
| C7—C8—H8 | 120.5 | C23—C29—H29B | 109.5 |
| C9—C8—H8 | 120.5 | H29A—C29—H29B | 109.5 |
| C10—C9—C8 | 120.3 (3) | C23—C29—H29C | 109.5 |
| C10—C9—H9 | 119.8 | H29A—C29—H29C | 109.5 |
| C8—C9—H9 | 119.8 | H29B—C29—H29C | 109.5 |
| C9—C10—C11 | 120.6 (4) | C25—C30—H30A | 109.5 |
| C9—C10—H10 | 119.7 | C25—C30—H30B | 109.5 |
| C11—C10—H10 | 119.7 | H30A—C30—H30B | 109.5 |
| N5—C11—C12 | 114.0 (3) | C25—C30—H30C | 109.5 |
| N5—C11—C10 | 126.1 (4) | H30A—C30—H30C | 109.5 |
| C12—C11—C10 | 119.8 (3) | H30B—C30—H30C | 109.5 |
| N4—C12—C11 | 119.3 (3) | N7i—Ni1—N7 | 180.0 |
| N4—C12—C7 | 121.0 (3) | N7i—Ni1—N8i | 94.38 (11) |
| C11—C12—C7 | 119.7 (3) | N7—Ni1—N8i | 85.62 (11) |
| O1—C13—C14 | 113.0 (4) | N7i—Ni1—N8 | 85.62 (11) |
| O1—C13—N5 | 124.7 (4) | N7—Ni1—N8 | 94.38 (11) |
| C14—C13—N5 | 122.2 (4) | N8i—Ni1—N8 | 179.998 (1) |
| C13—C14—N6 | 115.6 (4) | N7i—Ni1—N3i | 95.69 (12) |
| C13—C14—C15 | 123.8 (4) | N7—Ni1—N3i | 84.31 (12) |
| N6—C14—C15 | 119.8 (3) | N8i—Ni1—N3i | 90.98 (11) |
| C16—C15—C14 | 118.6 (4) | N8—Ni1—N3i | 89.02 (11) |
| C16—C15—H15 | 120.7 | N7i—Ni1—N3 | 84.31 (12) |
| C14—C15—H15 | 120.7 | N7—Ni1—N3 | 95.69 (12) |
| C17—C16—C15 | 123.1 (4) | N8i—Ni1—N3 | 89.02 (11) |
| C17—C16—H16 | 118.4 | N8—Ni1—N3 | 90.98 (11) |
| C15—C16—H16 | 118.4 | N3i—Ni1—N3 | 179.999 (1) |
| C16—C17—C22 | 119.2 (4) | C1—Fe1—C2 | 173.01 (14) |
| C16—C17—C18 | 121.3 (4) | C1—Fe1—C3 | 88.36 (15) |
| C22—C17—C18 | 119.5 (4) | C2—Fe1—C3 | 85.30 (15) |
| C17—C18—C19 | 119.7 (4) | C1—Fe1—N6 | 92.41 (13) |
| C17—C18—H18 | 120.2 | C2—Fe1—N6 | 88.74 (13) |
| C19—C18—H18 | 120.2 | C3—Fe1—N6 | 124.04 (17) |
| C20—C19—C18 | 119.9 (3) | C1—Fe1—N4 | 89.92 (13) |
| C20—C19—H19 | 120.0 | C2—Fe1—N4 | 91.90 (13) |
| C18—C19—H19 | 120.0 | C3—Fe1—N4 | 80.47 (16) |
| C19—C20—C21 | 120.8 (4) | N6—Fe1—N4 | 155.42 (12) |
| C19—C20—H20 | 119.6 | C1—Fe1—N5 | 95.05 (13) |
| C21—C20—H20 | 119.6 | C2—Fe1—N5 | 91.93 (13) |
| C22—C21—C20 | 119.4 (4) | C3—Fe1—N5 | 157.02 (16) |
| C22—C21—H21 | 120.3 | N6—Fe1—N5 | 78.60 (12) |
| C20—C21—H21 | 120.3 | N4—Fe1—N5 | 76.82 (12) |
| C21—C22—C17 | 120.7 (3) | C2—N3—Ni1 | 161.0 (3) |
| C21—C22—N6 | 119.9 (3) | C12—N4—C4 | 119.9 (3) |
| C17—C22—N6 | 119.4 (3) | C12—N4—Fe1 | 114.3 (2) |
| N7—C23—C24 | 109.1 (3) | C4—N4—Fe1 | 125.6 (2) |
| N7—C23—C28 | 111.5 (3) | C13—N5—C11 | 137.8 (3) |
| C24—C23—C28 | 108.6 (3) | C13—N5—Fe1 | 107.5 (2) |
| N7—C23—C29 | 110.0 (3) | C11—N5—Fe1 | 114.7 (2) |
| C24—C23—C29 | 111.4 (3) | C14—N6—C22 | 119.9 (3) |
| C28—C23—C29 | 106.2 (3) | C14—N6—Fe1 | 114.4 (2) |
| C25—C24—C23 | 120.8 (3) | C22—N6—Fe1 | 125.6 (2) |
| C25—C24—H24A | 107.1 | C23—N7—C27 | 116.8 (2) |
| C23—C24—H24A | 107.1 | C23—N7—Ni1 | 123.6 (2) |
| C25—C24—H24B | 107.1 | C27—N7—Ni1 | 105.0 (2) |
| C23—C24—H24B | 107.1 | C23—N7—H7 | 102.8 |
| H24A—C24—H24B | 106.8 | C27—N7—H7 | 102.8 |
| C24—C25—N8 | 109.9 (3) | Ni1—N7—H7 | 102.8 |
| C24—C25—C30 | 110.6 (3) | C26—N8—C25 | 115.7 (3) |
| N8—C25—C30 | 109.4 (3) | C26—N8—Ni1 | 106.2 (2) |
| C24—C25—H25 | 109.0 | C25—N8—Ni1 | 113.33 (18) |
| N8—C25—H25 | 109.0 | C26—N8—H8A | 107.1 |
| C30—C25—H25 | 109.0 | C25—N8—H8A | 107.1 |
| N8—C26—C27i | 110.9 (3) | Ni1—N8—H8A | 107.1 |
| N8—C26—H26A | 109.5 | H1WA—O1W—H1WB | 106 (3) |
| C27i—C26—H26A | 109.5 | H2WA—O2W—H2WB | 120 (4) |
| N4—C4—C5—C6 | 1.1 (6) | N5—Fe1—N4—C4 | 176.8 (3) |
| C4—C5—C6—C7 | 0.4 (6) | O1—C13—N5—C11 | −7.4 (8) |
| C5—C6—C7—C8 | −179.9 (4) | C14—C13—N5—C11 | 175.9 (4) |
| C5—C6—C7—C12 | −2.2 (6) | O1—C13—N5—Fe1 | 173.8 (4) |
| C12—C7—C8—C9 | 2.3 (6) | C14—C13—N5—Fe1 | −3.0 (5) |
| C6—C7—C8—C9 | 180.0 (4) | C12—C11—N5—C13 | 172.3 (4) |
| C7—C8—C9—C10 | −2.2 (6) | C10—C11—N5—C13 | −3.6 (7) |
| C8—C9—C10—C11 | 1.7 (6) | C12—C11—N5—Fe1 | −8.9 (4) |
| C9—C10—C11—N5 | 174.3 (4) | C10—C11—N5—Fe1 | 175.2 (3) |
| C9—C10—C11—C12 | −1.4 (6) | C1—Fe1—N5—C13 | 99.2 (3) |
| N5—C11—C12—N4 | 3.1 (5) | C2—Fe1—N5—C13 | −80.6 (3) |
| C10—C11—C12—N4 | 179.2 (3) | C3—Fe1—N5—C13 | −163.1 (4) |
| N5—C11—C12—C7 | −174.7 (4) | N6—Fe1—N5—C13 | 7.8 (3) |
| C10—C11—C12—C7 | 1.5 (6) | N4—Fe1—N5—C13 | −172.1 (3) |
| C8—C7—C12—N4 | −179.7 (3) | C1—Fe1—N5—C11 | −80.0 (3) |
| C6—C7—C12—N4 | 2.6 (6) | C2—Fe1—N5—C11 | 100.3 (3) |
| C8—C7—C12—C11 | −2.0 (6) | C3—Fe1—N5—C11 | 17.8 (5) |
| C6—C7—C12—C11 | −179.7 (4) | N6—Fe1—N5—C11 | −171.4 (3) |
| O1—C13—C14—N6 | 176.3 (3) | N4—Fe1—N5—C11 | 8.8 (3) |
| N5—C13—C14—N6 | −6.6 (6) | C13—C14—N6—C22 | −168.2 (4) |
| O1—C13—C14—C15 | 6.0 (6) | C15—C14—N6—C22 | 2.5 (5) |
| N5—C13—C14—C15 | −176.9 (4) | C13—C14—N6—Fe1 | 13.6 (5) |
| C13—C14—C15—C16 | 166.2 (4) | C15—C14—N6—Fe1 | −175.7 (3) |
| N6—C14—C15—C16 | −3.7 (6) | C21—C22—N6—C14 | 177.3 (4) |
| C14—C15—C16—C17 | 3.0 (7) | C17—C22—N6—C14 | −0.4 (6) |
| C15—C16—C17—C22 | −0.9 (7) | C21—C22—N6—Fe1 | −4.8 (5) |
| C15—C16—C17—C18 | −179.4 (4) | C17—C22—N6—Fe1 | 177.6 (3) |
| C16—C17—C18—C19 | 179.6 (4) | C1—Fe1—N6—C14 | −106.1 (3) |
| C22—C17—C18—C19 | 1.1 (7) | C2—Fe1—N6—C14 | 80.8 (3) |
| C17—C18—C19—C20 | −0.6 (7) | C3—Fe1—N6—C14 | 164.3 (3) |
| C18—C19—C20—C21 | −1.4 (6) | N4—Fe1—N6—C14 | −11.0 (5) |
| C19—C20—C21—C22 | 2.9 (6) | N5—Fe1—N6—C14 | −11.4 (3) |
| C20—C21—C22—C17 | −2.4 (6) | C1—Fe1—N6—C22 | 75.9 (3) |
| C20—C21—C22—N6 | −180.0 (3) | C2—Fe1—N6—C22 | −97.2 (3) |
| C16—C17—C22—C21 | −178.1 (4) | C3—Fe1—N6—C22 | −13.7 (4) |
| C18—C17—C22—C21 | 0.5 (6) | N4—Fe1—N6—C22 | 171.0 (3) |
| C16—C17—C22—N6 | −0.5 (6) | N5—Fe1—N6—C22 | 170.6 (3) |
| C18—C17—C22—N6 | 178.1 (4) | C24—C23—N7—C27 | 173.9 (3) |
| N7—C23—C24—C25 | −64.1 (4) | C28—C23—N7—C27 | −66.2 (4) |
| C28—C23—C24—C25 | 174.2 (3) | C29—C23—N7—C27 | 51.4 (4) |
| C29—C23—C24—C25 | 57.5 (4) | C24—C23—N7—Ni1 | 40.7 (4) |
| C23—C24—C25—N8 | 78.0 (4) | C28—C23—N7—Ni1 | 160.7 (2) |
| C23—C24—C25—C30 | −161.2 (3) | C29—C23—N7—Ni1 | −81.7 (3) |
| N2—C3—Fe1—C1 | −113.9 (7) | C26i—C27—N7—C23 | −178.6 (3) |
| N2—C3—Fe1—C2 | 63.2 (7) | C26i—C27—N7—Ni1 | −37.6 (3) |
| N2—C3—Fe1—N6 | −22.1 (7) | N8i—Ni1—N7—C23 | 150.3 (3) |
| N2—C3—Fe1—N4 | 156.0 (7) | N8—Ni1—N7—C23 | −29.7 (3) |
| N2—C3—Fe1—N5 | 147.1 (6) | N3i—Ni1—N7—C23 | −118.2 (3) |
| N7i—Ni1—N3—C2 | 33.8 (8) | N3—Ni1—N7—C23 | 61.8 (3) |
| N7—Ni1—N3—C2 | −146.2 (8) | N8i—Ni1—N7—C27 | 12.8 (2) |
| N8i—Ni1—N3—C2 | 128.3 (9) | N8—Ni1—N7—C27 | −167.2 (2) |
| N8—Ni1—N3—C2 | −51.7 (9) | N3i—Ni1—N7—C27 | 104.2 (2) |
| C11—C12—N4—C4 | −178.9 (3) | N3—Ni1—N7—C27 | −75.8 (2) |
| C7—C12—N4—C4 | −1.1 (5) | C27i—C26—N8—C25 | 167.7 (3) |
| C11—C12—N4—Fe1 | 4.6 (4) | C27i—C26—N8—Ni1 | 41.1 (3) |
| C7—C12—N4—Fe1 | −177.7 (3) | C24—C25—N8—C26 | 177.5 (3) |
| C5—C4—N4—C12 | −0.7 (5) | C30—C25—N8—C26 | 55.9 (4) |
| C5—C4—N4—Fe1 | 175.4 (3) | C24—C25—N8—Ni1 | −59.6 (3) |
| C1—Fe1—N4—C12 | 88.3 (3) | C30—C25—N8—Ni1 | 178.8 (2) |
| C2—Fe1—N4—C12 | −98.4 (3) | N7i—Ni1—N8—C26 | −15.4 (2) |
| C3—Fe1—N4—C12 | 176.7 (3) | N7—Ni1—N8—C26 | 164.6 (2) |
| N6—Fe1—N4—C12 | −7.3 (5) | N3i—Ni1—N8—C26 | −111.1 (2) |
| N5—Fe1—N4—C12 | −6.9 (2) | N3—Ni1—N8—C26 | 68.9 (2) |
| C1—Fe1—N4—C4 | −88.0 (3) | N7i—Ni1—N8—C25 | −143.4 (2) |
| C2—Fe1—N4—C4 | 85.3 (3) | N7—Ni1—N8—C25 | 36.6 (2) |
| C3—Fe1—N4—C4 | 0.4 (3) | N3i—Ni1—N8—C25 | 120.8 (2) |
| N6—Fe1—N4—C4 | 176.4 (3) | N3—Ni1—N8—C25 | −59.2 (2) |
Symmetry code: (i) −x+1, −y, −z+2.
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O2W—H2WA···O1 | 0.82 (2) | 2.14 (2) | 2.882 (5) | 151 (5) |
| O1W—H1WA···O2W | 0.85 (2) | 1.87 (7) | 2.623 (8) | 147 (11) |
| N8—H8A···O1Wii | 0.91 | 2.19 | 3.091 (7) | 169 |
Symmetry code: (ii) −x+1, y−1/2, −z+3/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: ZL2544).
References
- Brandenburg, K. (2006). DIAMOND Crystal Impact GbR, Bonn, Germany.
- Curtis, N. F. (1964). J. Chem. Soc. pp. 2644–2650.
- Higashi, T. (1995). ABSCOR Rigaku Coporation, Tokyo, Japan.
- Kim, J., Kwak, H. Y., Yoon, J. H., Ryu, D. W., Yoo, I. Y., Yang, N., Cho, B. K., Park, J. G., Lee, H. & Hong, C. S. (2009). Inorg. Chem. 48, 2956–2966. [DOI] [PubMed]
- Li, Y., Zhou, H. & Shen, X. (2012). Acta Cryst. E68, o1688. [DOI] [PMC free article] [PubMed]
- Liu, T., Zhang, Y. J., Kanegawa, S. & Sato, O. (2010). Angew. Chem. Int. Ed. 49, 8645–8648. [DOI] [PubMed]
- Panja, A., Guionneau, P., Jeon, I., Holmes, S. M., Clérac, R. & Mathonière, C. (2012). Inorg. Chem. 51, 12350–12359. [DOI] [PubMed]
- Rigaku (2008). CrystalClear Rigaku Corporation, Tokyo, Japan.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536813010234/zl2544sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813010234/zl2544Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report