Abstract
Numerous pathogens are transmitted from one host to another by hematophagous insect vectors. The interactions between a vector-borne organism and its vector vary in many ways, most of which are yet to be explored and identified. These interactions may play a role in the dynamics of the infection cycle. One way to evaluate these interactions is by studying the effects of the tested organism on the vector. In this study, we tested the effects of infection with Bartonella species on fitness-related variables of fleas by using Bartonella sp. strain OE 1-1, Xenopsylla ramesis fleas, and Meriones crassus jirds as a model system. Feeding parameters, including blood meal size and metabolic rate during digestion, as well as reproductive parameters, including fecundity, fertility, and life span, were compared between fleas experimentally infected with Bartonella and uninfected fleas. In addition, the developmental time, sex ratio, and body size of F1 offspring fleas were compared between the two groups. Most tested parameters did not differ between infected and uninfected fleas. However, F1 males produced by Bartonella-positive females were significantly smaller than F1 males produced by Bartonella-negative female fleas. The findings in this study suggest that bartonellae are well adapted to their flea vectors, and by minimally affecting their fitness they have evolved to better spread themselves in the natural environment.
INTRODUCTION
Numerous pathogens are transmitted from one host to another by hematophagous insect vectors. The interactions between pathogens and their vectors vary in many ways, most of which are yet to be explored and identified. These interactions may play a role in the dynamics of the infection cycle. Some vector-borne organisms do not rely on mechanical passage but undergo a period of growth, development, and reproduction within their vector (1). Even when the transmission of a pathogen by a vector is purely mechanical, the interactions with the pathogen may cause alterations in the vector's fitness-related characteristics. Plant and animal pathogens that alter the fitness of their insect vectors have recently been reviewed (2). It has been shown that the agents of epidemic typhus (Rickettsia prowazekii), bubonic plague (Yersinia pestis), leishmaniasis (Leishmania major and Leishmania infantum), onchocerciasis (Onchocerca spp.), filariasis (Brugia spp. and Dirofilaria immitis), malaria (Plasmodium spp.), mosquito-borne encephalitides, and African swine fever reduce the survival or fecundity of their vectors (3–13). Plasmodium and Leishmania infections represent the most-studied examples of pathogens affecting feeding and reproductive performance of their vectors (1). It has been reported that infection with Plasmodium parasites results in altered vector feeding behavior, mainly by prolonging host contact (1). In another study, infection with L. major or L. infantum significantly decreased the mean number of eggs produced by female sand flies (5). A definite effect on vector viability was observed when Y. pestis blocked the foregut of a flea, leading to its starvation and eventual death (14). Another bacterial organism, Rickettsia rickettsii, the agent of Rocky Mountain spotted fever, was reported to be lethal for Rocky Mountain wood ticks (Dermacentor andersoni) (15). Overall, 94.1% of nymphs infected as larvae died during molting into adults, and 88.3% of adult female ticks infected as nymphs died prior to feeding. Moreover, significantly fewer larvae developed from infected ticks. The lethal effects of R. rickettsii may explain the low prevalence of infected ticks in nature (15).
The spread rate of a vector-borne pathogen within a susceptible host population depends on several factors, including the vector survival, daily biting rate of the vector, the extrinsic incubation period, vector efficiency, the duration of infectivity, and the vector population size (2, 16). The pathogenic agent may alter some of these factors, and thus the specific interactions between a pathogen and its vector should be studied carefully in each system.
Organisms of the genus Bartonella are arthropod-borne, Gram-negative bacteria that infect mammalian erythrocytes and endothelial cells (17). Although Bartonella infections between humans and animals are important and widespread, information on the interactions of bartonellae with fleas is scarce. In particular, it is unknown whether bartonellae affect fitness-related parameters of their vectors. In order to fill this knowledge gap, we used Bartonella sp. strain OE 1-1, Xenopsylla ramesis fleas, and Meriones crassus jirds as a model system. Feeding and reproductive variables were compared between fleas experimentally infected with Bartonella and uninfected (naive) fleas. This study investigated whether bartonellae can affect the feeding and reproductive performance of their arthropod vector.
MATERIALS AND METHODS
Bartonella strain.
The inoculated isolate was obtained from a naturally infected wild M. crassus jird captured in the Negev Desert of Israel (18) and was cultured on chocolate agar plates incubated at 37°C with 5% CO2. Bacterial colonies were harvested into a solution containing 80% Luria-Bertani medium (Difco Microbiology, KS) and 20% glycerol and were frozen at −80°C until thawed and used for inoculation. The isolate used was confirmed by PCR and high-resolution melt (HRM) real-time PCR to be identical to Bartonella sp. OE 1-1, a strain closely related to Bartonella elizabethae.
Inoculation of jirds with Bartonella.
Ten naive female M. crassus (Sundevall's jirds), captured in the Negev Desert of Israel, were used. They were screened for Bartonella infection based on two consecutive blood cultures and PCR targeting the citrate synthase gene (gltA) as previously described (18), and they were confirmed to be free of Bartonella. Five jirds were subcutaneously inoculated along the dorsal midline between the scapulae with 107 CFU of Bartonella sp. OE 1-1 diluted in 1.0 ml phosphate-buffered saline (PBS). The other five naive jirds were used as negative controls and were injected subcutaneously with 1.0 ml PBS at the same injection site as the Bartonella-inoculated jirds. Three Bartonella-positive jirds and three Bartonella-negative jirds were used in the reproductive performance and life span trials. An additional two Bartonella-positive jirds and two Bartonella-negative jirds (five in total per trial) were used in the feeding and metabolic performance trials.
Fleas.
For feeding and metabolic performance trials, fleas were placed on all 10 rodents. For reproductive performance and life span trials, fleas were placed on 3 Bartonella-positive jirds (see Table 1, group 1) and on three Bartonella-negative jirds (see Table 1, group 2). The initial culture of Bartonella-free X. ramesis fleas was established on three Bartonella-free M. crassus jirds placed in separate individual cages. The cages contained a steel nest box with a screen floor and a pan with a mixture of autoclaved sand and dried bovine blood (examined by PCR to be Bartonella free) and maintained as described elsewhere (19, 20). Briefly, each jird was infested with 10 to 15 newly emerged fleas, and after 2 weeks, all substrate and bedding materials were collected from the cage and transferred into an incubator (FOC225E; Velp Scientifica srl, Milan, Italy), where flea emergence and development took place at 25°C with 75% relative humidity. Newly emerged fleas that developed from the substrate and bedding material were collected daily for 10 days and used in this study.
Table 1.
Reproductive performance for fleas that digested blood from Bartonella-positive or Bartonella-negative jirdsa
| Group no. | Jird no. | No. of female fleas (G0) | No. of eggs | No. of offspring (F1) | No. of eggs/flea | No. of offspring/egg |
|---|---|---|---|---|---|---|
| 1 | 1 | 12 | 17 | 14 | 1.42 | 0.82 |
| 2 | 14 | 22 | 17 | 1.57 | 0.77 | |
| 3 | 9 | 15 | 13 | 1.67 | 0.87 | |
| 2 | 4 | 7 | 11 | 8 | 1.57 | 0.73 |
| 5 | 13 | 24 | 20 | 1.85 | 0.83 | |
| 6 | 7 | 10 | 10 | 1.43 | 1.00 |
Data indicate the number of female fleas collected from jirds of the two groups, the number of eggs they produced, and the number of offspring. Group 1, fleas (G0) that digested blood from one of three Bartonella-negative jirds. Group 2, fleas (G0) that digested blood from one of three Bartonella-positive jirds.
Bartonella acquisition by fleas.
Seven to 14 newly emerged naive female X. ramesis (G0) fleas were placed on each of the five naive jirds and the five confirmed Bartonella sp. OE 1-1 experimentally infected jirds on day 75 postinoculation (see below). The procedure performed in this study ensured that fleas of a similar age were used in the experiments, as described elsewhere (21). Fleas fed on individual caged jirds that were restrained in a wire mesh tube (15-cm length and 5-cm diameter) to prevent self-grooming. After 2 h of feeding, female fleas were collected manually and separately from each jird and its cage. An additional feeding bout of 2 h was performed after 48 h. Following each feeding bout, measurements of feeding and metabolic performance were performed as described below.
Feeding and metabolic performance.
Evaluation of feeding performance was done by measuring the amount of blood taken by a flea during a single 2-hour feeding bout, and metabolic performance was determined by measuring the metabolic rate via the CO2 emission during blood digestion. Both measurements were done on the same day for the two feeding bouts. After feeding, fleas were collected into an Erlenmeyer glass flask, and the midgut of each flea was examined under light microscopy to verify whether a flea took a blood meal or not. The difference in mean body mass of a flea before and after feeding was calculated per mg body size of an unfed flea (i.e., prior to feeding) and was considered to be equal to the mean mass-specific amount of blood consumed. After the 2-h feeding bout, CO2 emission by fleas that took a blood meal was measured by using a flowthrough respirometry system (22). Since CO2 emissions of individual fleas were only slightly above the system baseline levels, fleas from each jird for a certain feeding bout were pooled (n = 7 to 10) and placed in a respirometer chamber. Variation in pool size reflected variability in feeding success on each jird. Carbon dioxide content (in ppm) of air exiting the respirometer chamber was sampled every 2 s for 30 min and measured with a CO2 analyzer (model 6262; Li-Cor, Lincoln, NE) in conjunction with data acquisition software (ExpeData, Sable Systems, Henderson, NV), as previously described (21–24). After 48 h, the fleas were placed again on the same jird as for the first bout, and a second CO2 emission measurement was performed.
Reproductive performance and life span.
The life span and variables associated with reproductive performance were measured and compared between Bartonella-positive and Bartonella-negative fleas. The variables included egg production (fecundity) and emerging adults (fertility) of G0 female fleas, as well as development time from eggs to adult, sex ratio, and femur length of F1 offspring as a measure of the flea body size (25). Female fleas collected from three Bartonella-negative jirds (see Table 1, jirds 1 to 3) and from three Bartonella-positive jirds (numbers 4 to 6) were placed separately in petri dishes. Ten newly emerged Bartonella-free male fleas (G0) that were separately fed for 2 h on Bartonella-negative jirds as described above were added to each petri dish in order to fertilize female fleas. Males were kept in petri dishes with female fleas for 15 h; thereafter, the petri dishes were checked for eggs, which were counted in each dish. The females (G0) from every petri dish were collected individually into a ventilated microtube (punched with a minor pin to allow air passage and prevent the flea from escaping) containing 0.5 mg of sand and were monitored daily to record their survival time. The males were collected into 70% alcohol for DNA extraction, as described below. After flea removal, the petri dishes containing eggs, flea feces, and flea gut voids were covered with a 3-mm layer of clean sand mixed with larvae medium (95% dry bovine blood, 4% millet flour, and 1% ground excrement of M. crassus) as described elsewhere (26). After 2 weeks, petri dishes with eggs were monitored daily for newly emerged fleas (F1). Every newly emerged flea was collected into a separate ventilated microtube containing 0.5 mg of sand and was monitored daily until death to record its survival time. After all the F1 fleas died, their sex was determined, and the lengths of right and left femurs of each flea were measured under a Stemi 2000-C stereomicroscope equipped with a digital camera (AxioCam ERc 5s), using the AxioVision 4 materials package extension image analysis setup (D; Carl Zeiss Microscopy, LLC, Thornwood, CA), at a magnification of ×40 and calibrated using an object micrometer.
Bartonella isolation and DNA extraction.
A 200-μl volume of blood was collected under isoflurane general anesthesia from the orbital sinus of each jird into an EDTA tube every 4 to 46 days (15 blood collections per jird) during the course of the study. Blood was cultured in an incubator on a chocolate agar plate for up to 6 weeks in 5% CO2 at 37°C. Bartonella colonies were diagnosed morphologically as small white creamy colonies. DNA was extracted from Bartonella colonies by using a DNA extraction kit (Illustra tissue minispin kit; GE Healthcare, Little Chalfont, United Kingdom) according to the manufacturer's instructions and confirmed using PCR and sequencing to be Bartonella sp. OE 1-1, as described below.
Confirmation of Bartonella acquisition by fleas.
After the measurement experiments, both parent and offspring fleas were placed in 70% alcohol until further analyzed for Bartonella. Each single flea was sliced to minute pieces by using a separate new sterile scalpel blade, and DNA was extracted from each single flea by using a DNA extraction kit (Illustra tissue minispin kit; GE Healthcare, Little Chalfont, United Kingdom) according to the manufacturer's instructions. Molecular screening for Bartonella was carried out with a conventional PCR targeting the 313-bp fragment of the gltA gene, using primers Bhcs.781p (GGGGACCAGCTCATGGTGG) and Bhcs.1137n (AATGCAAAAAGAACAGTAAACA) (27, 28). The PCR procedure was performed in a 50-μl reaction volume containing 2 μl of DNA, 20 pmol of each primer, 21 μl double-distilled water, and 25 μl of PCR-ready high-specificity reagent (Syntezza, Jerusalem, Israel). DNA extracted from cultured Bartonella Tel Aviv Rr strain (accession number FJ577651) was used as a positive control, and two samples containing all the ingredients of the reaction mixture except DNA were used as negative controls for all PCR tests. Positive blood cultures and fleas (at least 2 to 3 fleas), randomly chosen from each flea group, were selected for further sequence analysis. Amplicons were purified by using a PCR purification kit (ExoSAP-IT; USB, Cleveland, OH). DNA sequencing was carried out by utilizing a BigDye Terminator cycle sequencing chemistry 3700 DNA analyzer (Applied Biosystems [ABI], Foster City, CA) and the ABI data collection and sequence analysis software. Further analysis was performed using the Sequencher software, version 4.8 for Mac (Gene Codes Corporation, MI). The obtained sequences were analyzed initially by BLAST analysis through the NCBI Mega-BLAST algorithm and were further aligned with other Bartonella sequences.
Institutional animal care and use committee approvals.
This study was carried out in strict accordance with the recommendations of the National Institutes of Health's Guide for the Care and Use of Laboratory Animals (29) and approved by The Israel Nature and National Parks Protection Authority (approval number 2011/38279) and the Hebrew University Animal Care and Use Committee (approval number MD-10-12720-3).
Nucleotide sequence accession number.
The Bartonella sp. OE 1-1 citrate synthase gene (gltA) sequence was deposited in GenBank (accession number HM771297).
RESULTS
Bartonella infection of jirds and acquisition by fleas.
All 5 inoculated jirds became infected with Bartonella sp. OE 1-1, as confirmed by blood cultures and molecular screening of the colonies. Two feeding periods of 2 h each, 48 h apart, during the period when the jirds were bacteremic were sufficient for fleas to acquire Bartonella. Forty-one out of 55 (74%) individual female fleas collected from each Bartonella-infected jird acquired Bartonella sp. OE 1-1. All male fleas and all negative flea pools remained negative during the entire experiment. All obtained DNA sequences of amplified Bartonella from blood cultures and fleas were identical and were identified as Bartonella sp. OE 1-1.
Blood meal size.
There was no significant difference in blood meal size between fleas fed on Bartonella-positive and fleas fed on Bartonella-negative jirds (Mann-Whitney test, P = 0.0625 and P = 1.0 for the first and second blood meals, respectively). The mean blood meal size (of both first and second meals) of female flea feeding on a Bartonella-positive jird was 0.961 mg of blood per mg of starving flea, and that from a Bartonella-negative jird was 0.897 mg of blood per mg of starving flea (Mann-Whitney test, P = 0.4038). In both groups, the sizes of the first and second blood meals did not differ significantly (Wilcoxon matched pairs signed rank test, P = 0.222 and P = 0.3125, respectively).
Carbon dioxide emission during blood digestion.
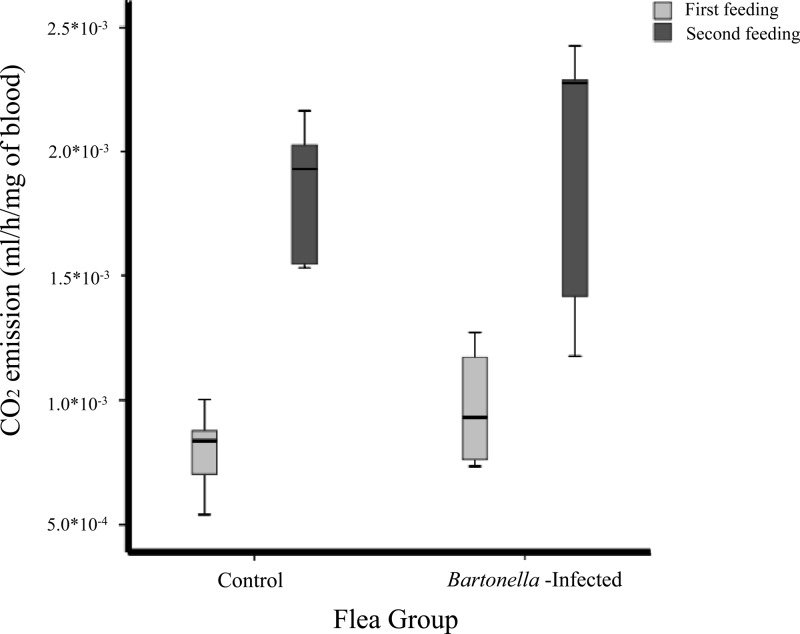
Comparison of CO2 emission from female fleas digesting blood from Bartonella-positive jirds and those digesting blood from Bartonella-negative jirds showed no significant difference between the two groups, either after the first or after the second blood meal (Mann-Whitney test, P = 0.413 P = 0.6905). In general, fleas in both groups emitted significantly more CO2 after the second meal than after the first blood meal (Wilcoxon signed rank test, P = 0.008) (Fig. 1).
Fig 1.
Carbon dioxide emission during flea blood digestion in two feeding periods. Bartonella-infected fleas were compared with noninfected fleas (G0).
Longevity, fecundity, and fertility of G0 female fleas.
No significant differences in longevity, the mean number of eggs, or the number of offspring produced per flea were found between Bartonella-positive and Bartonella-negative fleas (Table 1). The mean (± standard deviation) survival times of Bartonella-positive female fleas and Bartonella-negative female fleas were 15.7 ± 6.3 days and 15.3 ± 6.4 days, respectively (two-tailed t test, P = 0.7332). The average numbers of eggs produced by the Bartonella-positive group and the Bartonella-negative group were 1.553 ± 0.21 and 1.616 ± 0.12, respectively (Mann-Whitney test, P = 0.79). The mean fertility rates of the Bartonella-positive group and the Bartonella-negative group were 0.85 and 0.82 new offspring produced per egg, respectively (Pearson chi-square test, P = 0.9006).
Developmental time and longevity of F1 fleas.
No significant differences in developmental time from eggs to adults or longevity were found between the two groups. The mean developmental times of the Bartonella-negative group and the Bartonella-positive group were 47 and 48 days, respectively (t test, P = 0.605). The mean flea life spans of the Bartonella-negative group and the Bartonella-positive group were 33 ± 12.7 and 34 ± 11.1 days, respectively (t test, P = 0.582).
Sex ratios of F1 fleas.
Twenty-seven Bartonella-positive G0 female fleas produced 15 female and 23 male (F/M = 1.533) F1 fleas. Thirty-five Bartonella-negative G0 female fleas produced 21 female and 23 male (F/M = 1.095) F1 fleas. However, the difference between the F1 sex ratios in the two flea groups was not significant (Fisher's exact test, P = 0.5079).
Femur sizes of F1 fleas.
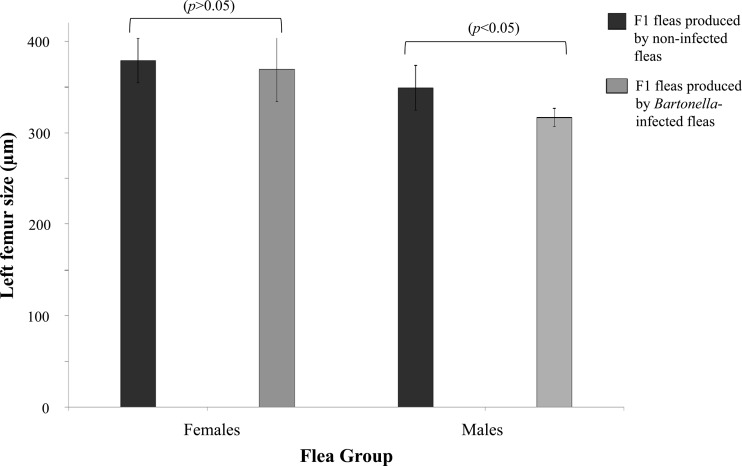
The lengths of the left and right femurs were measured for each of the 82 F1 fleas. No significant differences were detected between the lengths of the right and left femurs of the same individual flea in the negative males, negative females, positive males, or positive female F1 flea groups (paired t test, P = 0.883, 0.860, 0.826, and 0.966, respectively). Therefore, the left femur was measured and used as a proxy for body size for all comparisons. The mean left femurs of F1 males produced by positive G0 females (316.7 ± 9.8 μm) were significantly shorter than the mean left femurs of F1 males produced by negative G0 females (349.3 ± 24.2 μm; Mann-Whitney test, P < 0.0001). No difference was detected between mean left femurs of positive (369 ± 34.6 μm) and negative (379 ± 23.5 μm) F1 females (Mann-Whitney test, P = 0.822) (Fig. 2).
Fig 2.
Left femur length in F1 male and female fleas produced by Bartonella-infected and noninfected females. Note that the mean left femur of F1 male fleas produced by Bartonella-positive G0 females was significantly shorter than the mean left femur of F1 males produced by Bartonella-negative G0 females. No significant difference was detected between the mean left femurs of positive and negative F1 females.
DISCUSSION
Vector-pathogen interactions may be studied in different model systems. In this study, X. ramesis-Bartonella sp. OE 1-1 interactions were studied by comparing various fitness parameters between fleas that acquired Bartonella versus Bartonella-free fleas. A high percentage (74%) of female fleas acquired Bartonella sp. OE 1-1 after feeding on Bartonella-positive jirds. This high infection rate is comparable to that reported in other studies, where the Bartonella sp. OE 1-1 infection rate of X. ramesis fleas feeding on M. crassus jirds was 88% (30) and infection of Ctenophthalmus nobilis fleas feeding on Bartonella grahamii-infected or Bartonella taylorii-infected Myodes (Clethrionomys) glareolus voles was 100% (31). Two feeding periods of 2 h each, 48 h apart, were sufficient for fleas to acquire Bartonella. The 4-h feeding time should be considered rodent availability time rather than actual feeding time, due to the feeding pattern of fleas (i.e., feeding in several short sessions but not attached to the host). Thus, the design of this study was aimed at imitating the conditions under which flea inoculation with Bartonella in nature takes place. Our results demonstrated that Bartonella-infected fleas did not differ from Bartonella-free fleas in most of the tested feeding- and reproduction-related variables. These parameters have been shown previously to alter the performance of other pathogen-inoculated vectors compared to pathogen-free vectors (4, 5, 12, 32, 33).
It has been acknowledged that even a benign parasite hijacks part of the host's energy and that natural selection does not necessarily favor peaceful coexistence and might manifest in minor decreases in the host fitness (34). Our finding that Bartonella infection of mother fleas reduced the size of their male progeny and not that of their female progeny supports the latter suggestion of a minor impact on the host and its progeny, as an effect on the female progeny would have a greater impact on the proceeding generations. Flea body size might be considered an indicator of the “quality” of a flea, since a larger body size is associated with higher fecundity in insects within their species (35). It has recently been suggested that an increased body size in male fleas could increase their mating success and ultimately their fitness (36). A possible explanation for the smaller body size of male offspring (F1) may be a combination of a weaker provisioning of eggs by Bartonella-infected mother fleas and a higher sensitivity (less resistance) of male preimagoes to weaker mother egg provision; however, these speculations have to be further investigated.
Interestingly, fleas in our study emitted more CO2 in the second feeding period than in the first one, regardless of their infection status. The first feeding event is critical for fleas, as the majority of fleas are able to mate only after feeding. Newly emerged female fleas have been reported to have underdeveloped blocked ovaries, whereas newly emerged males have been reported to have a testicular plug that prevents the passage of sperm from the testes to the vas deferens (37). It was previously reported that X. ramesis fleas fed on female M. crassus jirds took a smaller first blood meal compared to their second and third meals and that this pattern was in contrast to fleas digesting blood from a male jird host (38). In our study, conducted on female jirds, although all fleas (from both groups) took a larger first blood meal, it was not significantly different from the amount taken during the second blood meal. However, after the second blood meal, all fleas used more energy. This might be explained by the possibility that in the second feeding period, fleas were already in the stage of egg production and probably had higher metabolic requirements compared with the first feeding period, when energy was used for blood consumption only.
In Aedes aegypti infected with Plasmodium gallinaceum, general metabolic rates are not different from noninfected mosquitoes except during blood digestion, when the metabolic rate is lower in infected mosquitoes (32). These results suggest that infection with P. gallinaceum does not lead to an increase in metabolic rate during midgut invasion and sporogony (32). The fact that no differences in blood meal size and metabolic rates between Bartonella-infected and noninfected fleas were detected in our study may be related to the specific Bartonella cycle within its flea vector, which is different from that of Plasmodium. It has been shown that Bartonella henselae can multiply in the digestive system of the cat flea and survive several days in flea feces (17). Additionally, Bartonella strains closely related to B. washoensis were detected in multiple tissues (hemolymph, midgut, and reproductive system) of several flea species collected from black-tailed prairie dogs (Cynomys ludovicianus), North American deer mice (Peromyscus maniculatus), and red foxes (Vulpes vulpes) (39). It seems that although Bartonella spp. multiply and circulate within their flea vectors, they do not cause alterations in the flea metabolism.
Our results indicate that infection with Bartonella does not affect fertility and fecundity of X. ramesis fleas, and no difference could be detected in the F1 sex ratio between the infected and noninfected flea groups. In contrast, in a study of vector fitness alterations with Western equine encephalitis virus (WEEV) and Culex tarsalis, the reproductive rate, measured in female eggs per female per generation, for the infected cohorts was significantly lower in the uninfected controls, whereas the reproductive rate in female eggs per female per day was higher in infected than uninfected cohorts (33).
No significant differences could be found in the time lag from hatching to emergence and in the life span of F1 X. ramesis fleas when comparing the Bartonella-infected and noninfected flea groups. In this regard, different contradicting results have been obtained in previous studies. Infection with Eastern equine encephalitis virus (EEEV) significantly reduced survival in Coquillettidia perturbans fed on viremic chicks compared with uninfected individuals in oral infection experiments. Intrathoracic infections of Coquillettidia perturbans did not reduce survival compared with diluent-inoculated groups, while infection with EEEV did not affect the survival of Aedes albopictus after oral infection or of Anopheles quadrimaculatus after either intrathoracic or oral infection (40). For malaria, different studies have indicated different effects of the parasite on its vector. A meta-analysis of 24 experiments showed that Plasmodium-infected mosquitoes had poorer survival rates than uninfected controls in 22 of the studies (41). However, mortality effects were more likely to be detected in unnatural vector-parasite combinations and in studies that followed the vectors for a longer period of time. Our study, conducted in a competent vector-pathogen system, demonstrated no such alterations. Additional investigations are required for further elucidation of bartonellae dynamics inside the flea vector.
Based on the results of this study, we concluded that infection of X. ramesis fleas with Bartonella sp. OE 1-1 altered neither the metabolic rate, blood consumption, life span, fertility and fecundity of the female flea (G0), nor the developmental time, the life span, or sex ratio of the F1 fleas. However, infection did alter the body size of F1 male fleas and contributed to a fitness reduction. These findings suggest that bartonellae are probably not pathogenic to fleas and are well adapted to them by minimally affecting their fitness parameters. This may indicate a long-term coevolution between fleas and bartonellae, allowing the latter to better spread themselves in an efficient way in the natural environment, as evidenced by the high prevalence of Bartonella infection in fleas worldwide.
ACKNOWLEDGMENTS
This research was supported by the Israel Science Foundation (grant number 30/11 to Shimon Harrus).
This is publication number 795 of the Mitrani Department of Desert Ecology.
Footnotes
Published ahead of print 29 March 2013
REFERENCES
- 1. Hurd H. 2003. Manipulation of medically important insect vectors by their parasites. Annu. Rev. Entomol. 48: 141– 161 [DOI] [PubMed] [Google Scholar]
- 2. Sisterson MS. 2009. Transmission of insect-vectored pathogens: effects of vector fitness as a function of infectivity status. Environ. Entomol. 38: 345– 355 [DOI] [PubMed] [Google Scholar]
- 3. Bacot AW, Martin CJ. 1914. Observations on the mechanism of the transmission of plague by fleas. J. Hyg. (Lond.) 13: 423– 439 [PMC free article] [PubMed] [Google Scholar]
- 4. Duke BO. 1962. Studies on factors influencing the transmission of onchocerciasis. I. The survival rate of Simulium damnosum under laboratory conditions and the effect upon it of Onchocerca volvulus. Ann. Trop. Med. Parasitol. 56: 130– 135 [PubMed] [Google Scholar]
- 5. El Sawaf BM, El Sattar SA, Shehata MG, Lane RP, Morsy TA. 1994. Reduced longevity and fecundity in Leishmania-infected sand flies. Am. J. Trop. Med. Hyg. 51: 767– 770 [DOI] [PubMed] [Google Scholar]
- 6. Faran ME, Turell MJ, Romoser WS, Routier RG, Gibbs PH, Cannon TL, Bailey CL. 1987. Reduced survival of adult Culex pipiens infected with Rift Valley fever virus. Am. J. Trop. Med. Hyg. 37: 403– 409 [DOI] [PubMed] [Google Scholar]
- 7. Hess WR, Endris RG, Lousa A, Caiado JM. 1989. Clearance of African swine fever virus from infected tick (Acari) colonies. J. Med. Entomol. 26: 314– 317 [DOI] [PubMed] [Google Scholar]
- 8. Husain A, Kershaw WE. 1971. The effect of filariasis on the ability of a vector mosquito to fly and feed and to transmit the infection. Trans. R. Soc. Trop. Med. Hyg. 65: 617– 619 [DOI] [PubMed] [Google Scholar]
- 9. Kershaw WE, Lavoipierre MM, Chalmers TA. 1953. Studies on the intake of microfilariae by their insect vectors, their survival, and their effect on the survival of their vectors. I. Dirofilaria immitis and Aedes aegypti. Ann. Trop. Med. Parasitol. 47: 207– 224 [DOI] [PubMed] [Google Scholar]
- 10. Maier WA, Becker-Feldman H, Seitz HM. 1987. Pathology of malaria-infected mosquitoes. Parasitol. Today 3: 216– 218 [DOI] [PubMed] [Google Scholar]
- 11. McGaw MM, Chandler LJ, Wasieloski LP, Blair CD, Beaty BJ. 1998. Effect of La Crosse virus infection on overwintering of Aedes triseriatus. Am. J. Trop. Med. Hyg. 58: 168– 175 [DOI] [PubMed] [Google Scholar]
- 12. Scott TW, Lorenz LH. 1998. Reduction of Culiseta melanura fitness by eastern equine encephalomyelitis virus. Am. J. Trop. Med. Hyg. 59: 341– 346 [DOI] [PubMed] [Google Scholar]
- 13. Snyder JC, Wheeler CM. 1945. The experimental infection of the human body louse, Pediculus humanus corporis, with murine and epidemic louse-borne typhus strains. J. Exp. Med. 82: 1– 20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hinnebusch BJ, Perry RD, Schwan TG. 1996. Role of the Yersinia pestis hemin storage (hms) locus in the transmission of plague by fleas. Science 273: 367– 370 [DOI] [PubMed] [Google Scholar]
- 15. Niebylski ML, Peacock MG, Schwan TG. 1999. Lethal effect of Rickettsia rickettsii on its tick vector (Dermacentor andersoni). Appl. Environ. Microbiol. 65: 773– 778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Macdonald G. 1961. Epidemiologic models in studies of vector borne diseases. Public Health Rep. 76: 753– 764 [PMC free article] [PubMed] [Google Scholar]
- 17. Chomel BB, Boulouis HJ, Breitschwerdt EB, Kasten RW, Vayssier-Taussat M, Birtles RJ, Koehler JE, Dehio C. 2009. Ecological fitness and strategies of adaptation of Bartonella species to their hosts and vectors. Vet. Res. 40: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morick D, Krasnov BR, Khokhlova IS, Gottlieb Y, Harrus S. 2011. Investigation of Bartonella acquisition and transmission in Xenopsylla ramesis fleas (Siphonaptera: Pulicidae). Mol. Ecol. 20:2864– 2870 [DOI] [PubMed] [Google Scholar]
- 19. Krasnov BR, Khokhlova IS, Fielden LJ, Burdelova NV. 2001. Development rates of two Xenopsylla flea species in relation to air temperature and humidity. Med. Vet. Entomol. 15: 249– 258 [DOI] [PubMed] [Google Scholar]
- 20. Krasnov BR, Khokhlova IS, Fielden LJ, Burdelova NV. 2001. Effect of air temperature and humidity on the survival of pre-imaginal stages of two flea species (Siphonaptera: Pulicidae). J. Med. Entomol. 38: 629– 637 [DOI] [PubMed] [Google Scholar]
- 21. Khokhlova IS, Fielden LJ, Degen AA, Krasnov BR. 2012. Digesting blood of an auxiliary host in fleas: effect of phylogenetic distance from a principal host. J. Exp. Biol. 215: 1259– 1265 [DOI] [PubMed] [Google Scholar]
- 22. Fielden LJ, Krasnov BR, Khokhlova IS, Arakelyan MS. 2004. Respiratory gas exchange in the desert flea Xenopsylla ramesis (Siphonaptera: Pulicidae): response to temperature and blood-feeding. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 137: 557– 565 [DOI] [PubMed] [Google Scholar]
- 23. Krasnov BR, Khokhlova IS, Burdelov SA, Fielden LJ. 2004. Metabolic rate and jump performance in seven species of desert fleas. J. Insect Physiol. 50: 149– 156 [DOI] [PubMed] [Google Scholar]
- 24. Sarfati M, Krasnov BR, Ghazaryan L, Khokhlova IS, Fielden LJ, Degen AA. 2005. Energy costs of blood digestion in a host-specific haematophagous parasite. J. Exp. Biol. 208: 2489– 2496 [DOI] [PubMed] [Google Scholar]
- 25. Krasnov BR, Burdelov SA, Khokhlova IS, Burdelova NV. 2004. Sexual size dimorphism, morphological traits and jump performance in seven species of desert fleas (Siphonaptera). J. Zool. 261: 181– 189 [Google Scholar]
- 26. Krasnov BR, Burdelova NV, Khokhlova IS, Shenbrot GI, Degen A. 2005. Larval interspecific competition in two flea species parasitic on the same rodent host. Ecol. Entomol. 30: 146– 155 [Google Scholar]
- 27. Relman DA, Loutit JS, Schmidt TM, Falkow S, Tompkins LS. 1990. The agent of bacillary angiomatosis. An approach to the identification of uncultured pathogens. N. Engl. J. Med. 323: 1573– 1580 [DOI] [PubMed] [Google Scholar]
- 28. Norman AF, Regnery R, Jameson P, Greene C, Krause DC. 1995. Differentiation of Bartonella-like isolates at the species level by PCR-restriction fragment length polymorphism in the citrate synthase gene. J. Clin. Microbiol. 33: 1797– 1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. National Research Council 1996. Guide for the care and use of laboratory animals. National Academies Press, Washington, DC [Google Scholar]
- 30.Morick D, Krasnov BR, Khokhlova IS, Gottlieb Y, Harrus S. 2013. Transmission dynamics of Bartonella species OE 1-1 in Sundevall's jirds (Meriones crassus). Appl. Environ. Microbiol. 79: 1258– 1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bown KJ, Bennet M, Begon M. 2004. Flea-borne Bartonella grahamii and Bartonella taylorii in bank voles. Emerg. Infect. Dis. 10: 684– 687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gray EM, Bradley TJ. 2006. Malarial infection in Aedes aegypti: effects on feeding, fecundity and metabolic rate. Parasitology 132: 169– 176 [DOI] [PubMed] [Google Scholar]
- 33. Mahmood F, Reisen WK, Chiles RE, Fang Y. 2004. Western equine encephalomyelitis virus infection affects the life table characteristics of Culex tarsalis (Diptera: Culicidae). J. Med. Entomol. 41: 982– 986 [DOI] [PubMed] [Google Scholar]
- 34. Ewald PW. 1995. The evolution of virulence: a unifying link between parasitology and ecology. J. Parasitol. 81: 659– 669 [PubMed] [Google Scholar]
- 35. Honeǩ A. 1993. Intraspecific variation in body size and fecundity in insects: a general relationship. Oikos 66: 483– 492 [Google Scholar]
- 36. Khokhlova IS, Serobyan V, Degen AA, Krasnov BR. 2010. Host gender and offspring quality in a flea parasitic on a rodent. J. Exp. Biol. 213: 3299– 3304 [DOI] [PubMed] [Google Scholar]
- 37. Dean SR, Meola RW. 1997. Effect of juvenile hormone and juvenile hormone mimics on sperm transfer from the testes of the male cat flea (Siphonaptera: Pulicidae). J. Med. Entomol. 34: 485– 488 [DOI] [PubMed] [Google Scholar]
- 38. Khokhlova IS, Serobyan V, Krasnov BR, Degen AA. 2009. Is the feeding and reproductive performance of the flea, Xenopsylla ramesis, affected by the gender of its rodent host, Meriones crassus? J. Exp. Biol. 212: 1429– 1435 [DOI] [PubMed] [Google Scholar]
- 39. Brinkerhoff RJ, Kabeya H, Inoue K, Bai Y, Maruyama S. 2010. Detection of multiple Bartonella species in digestive and reproductive tissues of fleas collected from sympatric mammals. ISME J. 4: 955– 958 [DOI] [PubMed] [Google Scholar]
- 40. Moncayo AC, Edman JD, Turell MJ. 2000. Effect of Eastern equine encephalomyelitis virus on the survival of Aedes albopictus, Anopheles quadrimaculatus, and Coquillettidia perturbans (Diptera: Culicidae). J. Med. Entomol. 37: 701– 706 [DOI] [PubMed] [Google Scholar]
- 41. Ferguson HM, Read AF. 2002. Why is the effect of malaria parasites on mosquito survival still unresolved? Trends Parasitol. 18: 256– 261 [DOI] [PubMed] [Google Scholar]