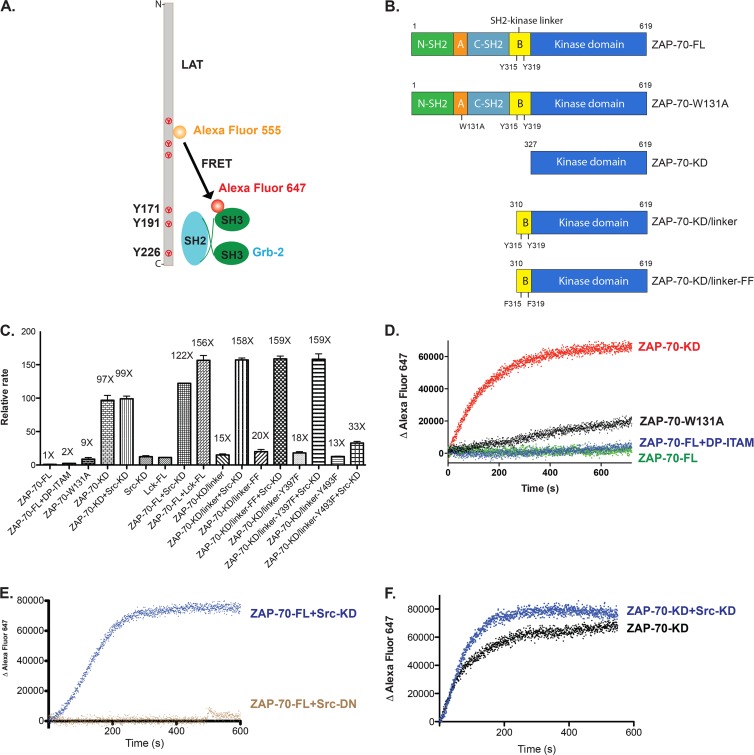
Fig 9.
Phosphorylation of ZAP-70 by Src family kinases fully activates ZAP-70 in vitro. (A) In vitro FRET-based approach to measure the catalytic activity of ZAP-70 for its physiological substrate, LAT. (B) Domain architectures of various ZAP-70 truncation constructs. (C) Relative rates of phosphorylation of LAT by ZAP-70 or Src family kinases. Src-KD, isolated c-Src kinase domain; Lck-FL, full-length Lck; DP-ITAM, doubly phosphorylated ITAM peptide. (D) Time course of phosphorylation of LAT as measured by the recruitment of Grb2. Red, isolated ZAP-70 kinase domain; black, ZAP-70-W131A; blue, full-length ZAP-70 bound to an excess amount of doubly phosphorylated ITAM peptide; green, full-length ZAP-70. (E) Time course of phosphorylation of LAT as measured by the recruitment of Grb2. Blue, full-length ZAP-70 plus Src kinase domain; brown, full-length ZAP-70 plus Src kinase domain with D386N mutation. (F) Time course of phosphorylation of LAT as measured by the recruitment of Grb2. Black, isolated ZAP-70 kinase domain; blue, isolated ZAP-70 kinase domain plus Src kinase domain.