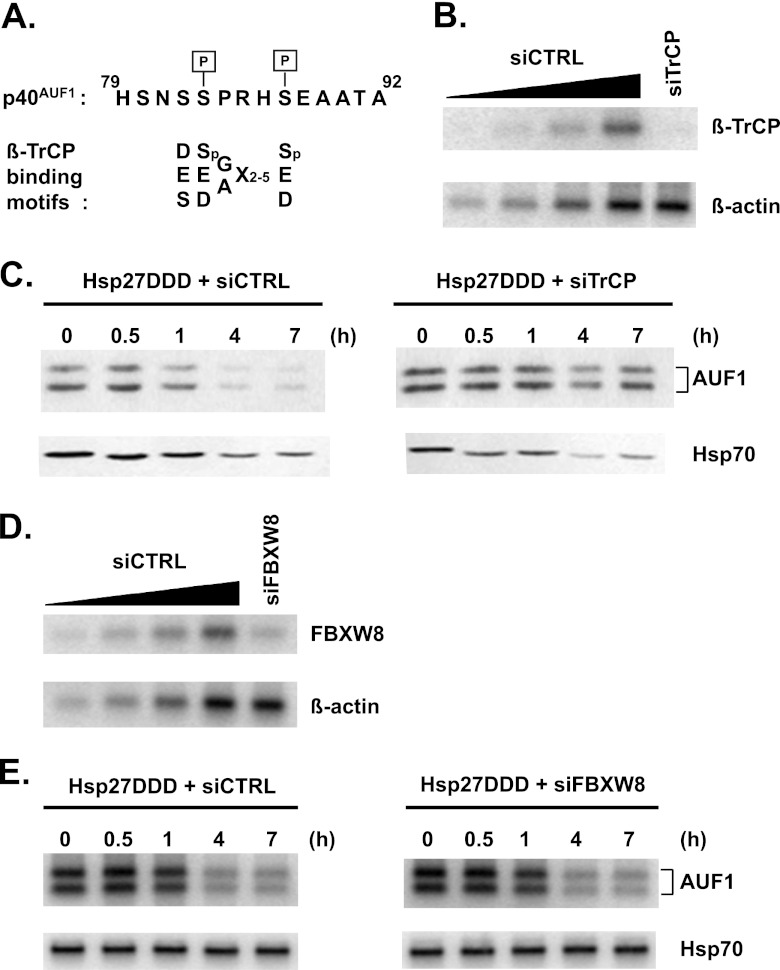
Fig 2.
Phosphomimetic Hsp27 promotes AUF1 degradation via the F-box protein β-TrCP. (A) Alignment of AUF1 amino acid sequence with β-TrCP binding motifs. Shown on top, in single-letter codes, is a portion of the 19-amino-acid sequence encoded by exon 2 that distinguishes the p40/p45 AUF1 isoforms from the p37/p42 isoforms. The two indicated serines, Ser83 and Ser87, are phosphorylated in cellular p40AUF1 (33, 34). Shown below the AUF1 sequence are consensus binding motifs within β-TrCP substrates, with the phosphorylated serines in the canonical motif, DSGX2–5S, indicated as Sp (29). (B) HeLa/Hsp27DDD cells were transfected with control siRNA (siCTRL) or siRNA targeted to βTrCP1/2 (siTrCP). After 2 days, extracts were prepared for Western blot analyses of β-TrCP1/2 and β-actin (loading control). A 2-fold dilution series of extract from siCTRL-transfected cells (lanes 1 to 4) permitted estimates of β-TrCP knockdown efficiency (lane 5). (C) Knockdown of β-TrCP1/2 stabilizes AUF1. HeLa/Hsp27DDD cells were transfected with siCTRL (left panel) or siTrCP (right panel). After 2 days, cells were cultured with cycloheximide for protein stability assays by Western blotting analyses with antibodies to AUF1 and Hsp70 (as a control). (D) HeLa/Hsp27DDD cells were transfected with control siRNA (siCTRL) or siRNA targeted to FBXW8 (siFBXW8). After 2 days, extracts were prepared for Western blot analyses of FBXW8 and β-actin (loading control). A 2-fold dilution series of extract from siCTRL-transfected cells (lanes 1 to 4) permitted estimates of FBXW8 knockdown efficiency (lane 5). (E) Knockdown of FBXW8 has no effect on AUF1 protein stability. HeLa/Hsp27DDD cells were transfected with siCTRL (left panel) or siFBXW8 (right panel). After 2 days, cells were cultured with cycloheximide for protein stability assays by Western blotting analyses with antibodies to AUF1 and Hsp70 (as a control).