Abstract
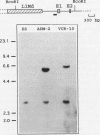
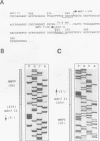
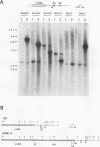
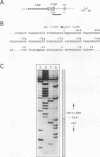
In multidrug-resistant (MDR) derivatives of the mouse lymphoid tumor P388, the emergence of MDR is associated with overexpression and transcriptional activation of the mdr3 gene, either in the absence of (P388/VCR-10) or concomitant with (P388/ADM-2) gene amplification. In both instances, Northern (RNA) blotting analyses have suggested the presence of altered mdr3 transcripts in these cells, possibly originating from novel transcription initiation sites. The mechanisms underlying mdr3 overexpression in these cells have been investigated. In P388/VCR-10 cells, Southern blotting analyses together with genomic DNA cloning and nucleotide sequencing have demonstrated the presence of an intact mouse mammary tumor virus (MMTV) within the boundaries of intron 1 of mdr3. cDNA cloning and nucleotide sequencing indicated that this integration event results in the synthesis and overexpression of a hybrid MMTV-mdr3 mRNA which initiates within the U3 region of the 5' long terminal repeat (LTR) of the provirus. Consequently, this mRNA lacks the normal exon 1 of mdr3 but contains (i) MMTV LTR-derived sequences at its 5' end, (ii) a novel mdr3 exon, mapping within the boundaries of intron 1 downstream of the MMTV integration site and generated by alternative splicing, and (iii) an otherwise intact 3' portion of mdr3 starting at exon 2. A similar type of analysis of P388/ADM-2 cells revealed that mdr3 overexpression in these cells is associated with the integration of an intracisternal A particle (IAP) within an L1Md repetitive element, immediately upstream of mdr3. The IAP insertion results in the overexpression of hybrid IAP-mdr3 mRNA transcripts that initiate within the 3' LTR of the IAP and which contain IAP LTR-derived sequences at the 5' end spliced 14 nucleotides upstream of the normal exon 1 of mdr3. Taken together, these results indicate that independent retroviral insertions were the initial mutagenic event responsible for mdr3 overexpression and survival during drug selection of these cell lines. Amplification of the rearranged and activated mdr3 gene copy occurred during further selection for high-level drug resistance in P388/ADM-2 cells.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Algate P. A., McCubrey J. A. Autocrine transformation of hemopoietic cells resulting from cytokine message stabilization after intracisternal A particle transposition. Oncogene. 1993 May;8(5):1221–1232. [PubMed] [Google Scholar]
- Arceci R. J., Baas F., Raponi R., Horwitz S. B., Housman D., Croop J. M. Multidrug resistance gene expression is controlled by steroid hormones in the secretory epithelium of the uterus. Mol Reprod Dev. 1990 Feb;25(2):101–109. doi: 10.1002/mrd.1080250202. [DOI] [PubMed] [Google Scholar]
- Blankenstein T., Qin Z. H., Li W. Q., Diamantstein T. DNA rearrangement and constitutive expression of the interleukin 6 gene in a mouse plasmacytoma. J Exp Med. 1990 Mar 1;171(3):965–970. doi: 10.1084/jem.171.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt C., Aberdam D., Schwartz R., Sachs L. DNA rearrangement of a homeobox gene in myeloid leukaemic cells. EMBO J. 1988 Dec 20;7(13):4283–4290. doi: 10.1002/j.1460-2075.1988.tb03326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bénard J., Da Silva J., Teyssier J. R., Riou G. Over-expression of MDR1 gene with no DNA amplification in a multiple-drug-resistant human ovarian carcinoma cell line. Int J Cancer. 1989 Mar 15;43(3):471–477. doi: 10.1002/ijc.2910430322. [DOI] [PubMed] [Google Scholar]
- Chaudhary P. M., Roninson I. B. Induction of multidrug resistance in human cells by transient exposure to different chemotherapeutic drugs. J Natl Cancer Inst. 1993 Apr 21;85(8):632–639. doi: 10.1093/jnci/85.8.632. [DOI] [PubMed] [Google Scholar]
- Chen C. J., Chin J. E., Ueda K., Clark D. P., Pastan I., Gottesman M. M., Roninson I. B. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986 Nov 7;47(3):381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- Chin K. V., Chauhan S. S., Pastan I., Gottesman M. M. Regulation of mdr RNA levels in response to cytotoxic drugs in rodent cells. Cell Growth Differ. 1990 Aug;1(8):361–365. [PubMed] [Google Scholar]
- Chin K. V., Tanaka S., Darlington G., Pastan I., Gottesman M. M. Heat shock and arsenite increase expression of the multidrug resistance (MDR1) gene in human renal carcinoma cells. J Biol Chem. 1990 Jan 5;265(1):221–226. [PubMed] [Google Scholar]
- Chin K. V., Ueda K., Pastan I., Gottesman M. M. Modulation of activity of the promoter of the human MDR1 gene by Ras and p53. Science. 1992 Jan 24;255(5043):459–462. doi: 10.1126/science.1346476. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Christy R. J., Huang R. C. Functional analysis of the long terminal repeats of intracisternal A-particle genes: sequences within the U3 region determine both the efficiency and direction of promoter activity. Mol Cell Biol. 1988 Mar;8(3):1093–1102. doi: 10.1128/mcb.8.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausse N., Baines D., Moore R., Brookes S., Dickson C., Peters G. Activation of both Wnt-1 and Fgf-3 by insertion of mouse mammary tumor virus downstream in the reverse orientation: a reappraisal of the enhancer insertion model. Virology. 1993 May;194(1):157–165. doi: 10.1006/viro.1993.1245. [DOI] [PubMed] [Google Scholar]
- Cohen D., Higman S. M., Hsu S. I., Horwitz S. B. The involvement of a LINE-1 element in a DNA rearrangement upstream of the mdr1a gene in a taxol multidrug-resistant murine cell line. J Biol Chem. 1992 Oct 5;267(28):20248–20254. [PubMed] [Google Scholar]
- Cohen D., Piekarz R. L., Hsu S. I., DePinho R. A., Carrasco N., Horwitz S. B. Structural and functional analysis of the mouse mdr1b gene promoter. J Biol Chem. 1991 Feb 5;266(4):2239–2244. [PubMed] [Google Scholar]
- Cohen J. B., Unger T., Rechavi G., Canaani E., Givol D. Rearrangement of the oncogene c-mos in mouse myeloma NSI and hybridomas. Nature. 1983 Dec 22;306(5945):797–799. doi: 10.1038/306797a0. [DOI] [PubMed] [Google Scholar]
- Corrias M. V., Cornaglia-Ferraris P., Di Martino D., Stenger A. M., Lanino E., Boni L., Tonini G. P. Expression of multiple drug resistance gene, MDR1, and N-myc oncogene in an Italian population of human neuroblastoma patients. Anticancer Res. 1990 Jul-Aug;10(4):897–902. [PubMed] [Google Scholar]
- Dano K. Active outward transport of daunomycin in resistant Ehrlich ascites tumor cells. Biochim Biophys Acta. 1973 Oct 25;323(3):466–483. doi: 10.1016/0005-2736(73)90191-0. [DOI] [PubMed] [Google Scholar]
- Devault A., Gros P. Two members of the mouse mdr gene family confer multidrug resistance with overlapping but distinct drug specificities. Mol Cell Biol. 1990 Apr;10(4):1652–1663. doi: 10.1128/mcb.10.4.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Smith R., Brookes S., Peters G. Proviral insertions within the int-2 gene can generate multiple anomalous transcripts but leave the protein-coding domain intact. J Virol. 1990 Feb;64(2):784–793. doi: 10.1128/jvi.64.2.784-793.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Smith R., Brookes S., Peters G. Tumorigenesis by mouse mammary tumor virus: proviral activation of a cellular gene in the common integration region int-2. Cell. 1984 Jun;37(2):529–536. doi: 10.1016/0092-8674(84)90383-0. [DOI] [PubMed] [Google Scholar]
- Dudley J. P. Mouse mammary tumor proviruses from a T-cell lymphoma are associated with the retroposon L1Md. J Virol. 1988 Feb;62(2):472–478. doi: 10.1128/jvi.62.2.472-478.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Falzon M., Kuff E. L. Multiple protein-binding sites in an intracisternal A particle long terminal repeat. J Virol. 1988 Nov;62(11):4070–4077. doi: 10.1128/jvi.62.11.4070-4077.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith M. E., Madden M. J., Morrow C. S., Cowan K. H. A Y-box consensus sequence is required for basal expression of the human multidrug resistance (mdr1) gene. J Biol Chem. 1993 Mar 15;268(8):5856–5860. [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Gros P., Ben Neriah Y. B., Croop J. M., Housman D. E. Isolation and expression of a complementary DNA that confers multidrug resistance. Nature. 1986 Oct 23;323(6090):728–731. doi: 10.1038/323728a0. [DOI] [PubMed] [Google Scholar]
- Gros P., Croop J., Housman D. Mammalian multidrug resistance gene: complete cDNA sequence indicates strong homology to bacterial transport proteins. Cell. 1986 Nov 7;47(3):371–380. doi: 10.1016/0092-8674(86)90594-5. [DOI] [PubMed] [Google Scholar]
- Gros P., Raymond M., Bell J., Housman D. Cloning and characterization of a second member of the mouse mdr gene family. Mol Cell Biol. 1988 Jul;8(7):2770–2778. doi: 10.1128/mcb.8.7.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley R. G., Shulman M. J., Hozumi N. Transposition of two different intracisternal A particle elements into an immunoglobulin kappa-chain gene. Mol Cell Biol. 1984 Dec;4(12):2565–2572. doi: 10.1128/mcb.4.12.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberlein C., Kawai M., Franz M. J., Beck-Engeser G., Daniel C. P., Ostertag W., Stocking C. Retrotransposons as mutagens in the induction of growth autonomy in hematopoietic cells. Oncogene. 1990 Dec;5(12):1799–1807. [PubMed] [Google Scholar]
- Herzog C. E., Tsokos M., Bates S. E., Fojo A. T. Increased mdr-1/P-glycoprotein expression after treatment of human colon carcinoma cells with P-glycoprotein antagonists. J Biol Chem. 1993 Feb 5;268(4):2946–2952. [PubMed] [Google Scholar]
- Holmes J., Jacobs A., Carter G., Janowska-Wieczorek A., Padua R. A. Multidrug resistance in haemopoietic cell lines, myelodysplastic syndromes and acute myeloblastic leukaemia. Br J Haematol. 1989 May;72(1):40–44. doi: 10.1111/j.1365-2141.1989.tb07649.x. [DOI] [PubMed] [Google Scholar]
- Horowitz M., Luria S., Rechavi G., Givol D. Mechanism of activation of the mouse c-mos oncogene by the LTR of an intracisternal A-particle gene. EMBO J. 1984 Dec 1;3(12):2937–2941. doi: 10.1002/j.1460-2075.1984.tb02235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. I., Cohen D., Kirschner L. S., Lothstein L., Hartstein M., Horwitz S. B. Structural analysis of the mouse mdr1a (P-glycoprotein) promoter reveals the basis for differential transcript heterogeneity in multidrug-resistant J774.2 cells. Mol Cell Biol. 1990 Jul;10(7):3596–3606. doi: 10.1128/mcb.10.7.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. I., Lothstein L., Horwitz S. B. Differential overexpression of three mdr gene family members in multidrug-resistant J774.2 mouse cells. Evidence that distinct P-glycoprotein precursors are encoded by unique mdr genes. J Biol Chem. 1989 Jul 15;264(20):12053–12062. [PubMed] [Google Scholar]
- Ikeguchi M., Teeter L. D., Eckersberg T., Ganapathi R., Kuo M. T. Structural and functional analyses of the promoter of the murine multidrug resistance gene mdr3/mdr1a reveal a negative element containing the AP-1 binding site. DNA Cell Biol. 1991 Nov;10(9):639–649. doi: 10.1089/dna.1991.10.639. [DOI] [PubMed] [Google Scholar]
- Ito Y., Tanimoto M., Kumazawa T., Okumura M., Morishima Y., Ohno R., Saito H. Increased P-glycoprotein expression and multidrug-resistant gene (mdr1) amplification are infrequently found in fresh acute leukemia cells. Sequential analysis of 15 cases at initial presentation and relapsed stage. Cancer. 1989 Apr 15;63(8):1534–1538. doi: 10.1002/1097-0142(19890415)63:8<1534::aid-cncr2820630813>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Kohno K., Sato S., Takano H., Matsuo K., Kuwano M. The direct activation of human multidrug resistance gene (MDR1) by anticancer agents. Biochem Biophys Res Commun. 1989 Dec 29;165(3):1415–1421. doi: 10.1016/0006-291x(89)92761-7. [DOI] [PubMed] [Google Scholar]
- Kuff E. L., Lueders K. K. The intracisternal A-particle gene family: structure and functional aspects. Adv Cancer Res. 1988;51:183–276. doi: 10.1016/s0065-230x(08)60223-7. [DOI] [PubMed] [Google Scholar]
- Kung H. J., Boerkoel C., Carter T. H. Retroviral mutagenesis of cellular oncogenes: a review with insights into the mechanisms of insertional activation. Curr Top Microbiol Immunol. 1991;171:1–25. doi: 10.1007/978-3-642-76524-7_1. [DOI] [PubMed] [Google Scholar]
- Kuo M. T., Zhao J. Y., Teeter L. D., Ikeguchi M., Chisari F. V. Activation of multidrug resistance (P-glycoprotein) mdr3/mdr1a gene during the development of hepatocellular carcinoma in hepatitis B virus transgenic mice. Cell Growth Differ. 1992 Aug;3(8):531–540. [PubMed] [Google Scholar]
- Lemontt J. F., Azzaria M., Gros P. Increased mdr gene expression and decreased drug accumulation in multidrug-resistant human melanoma cells. Cancer Res. 1988 Nov 15;48(22):6348–6353. [PubMed] [Google Scholar]
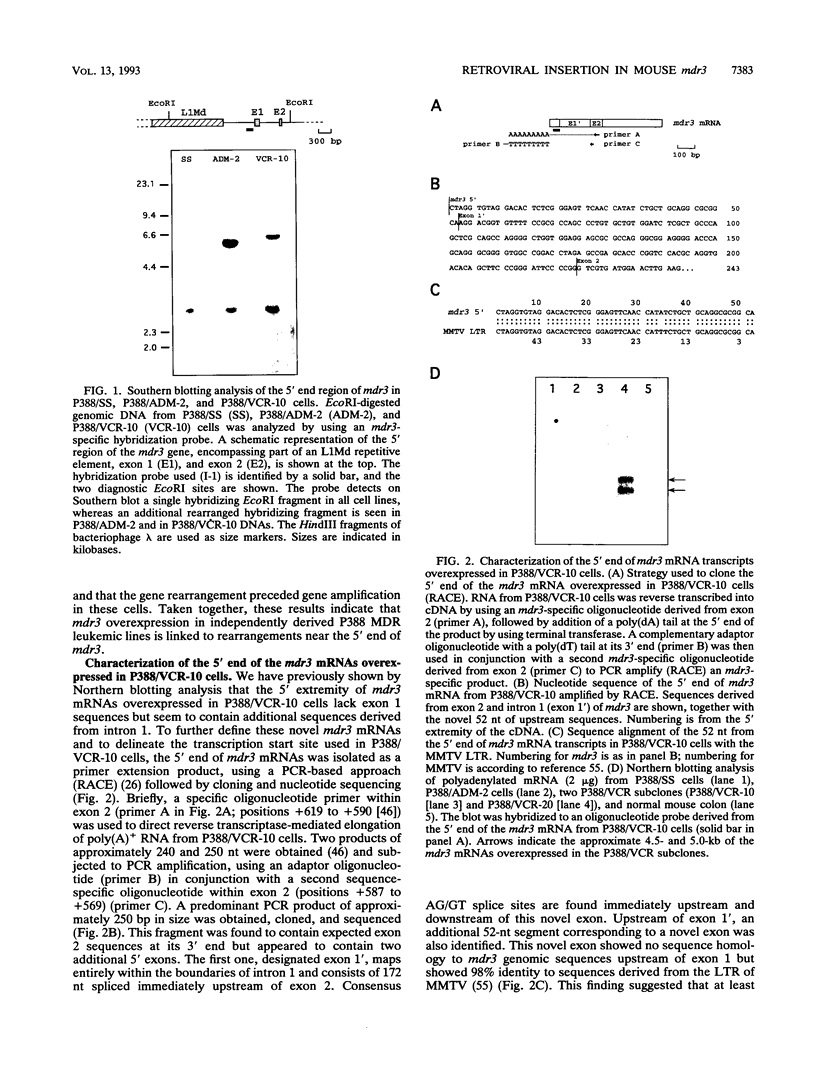
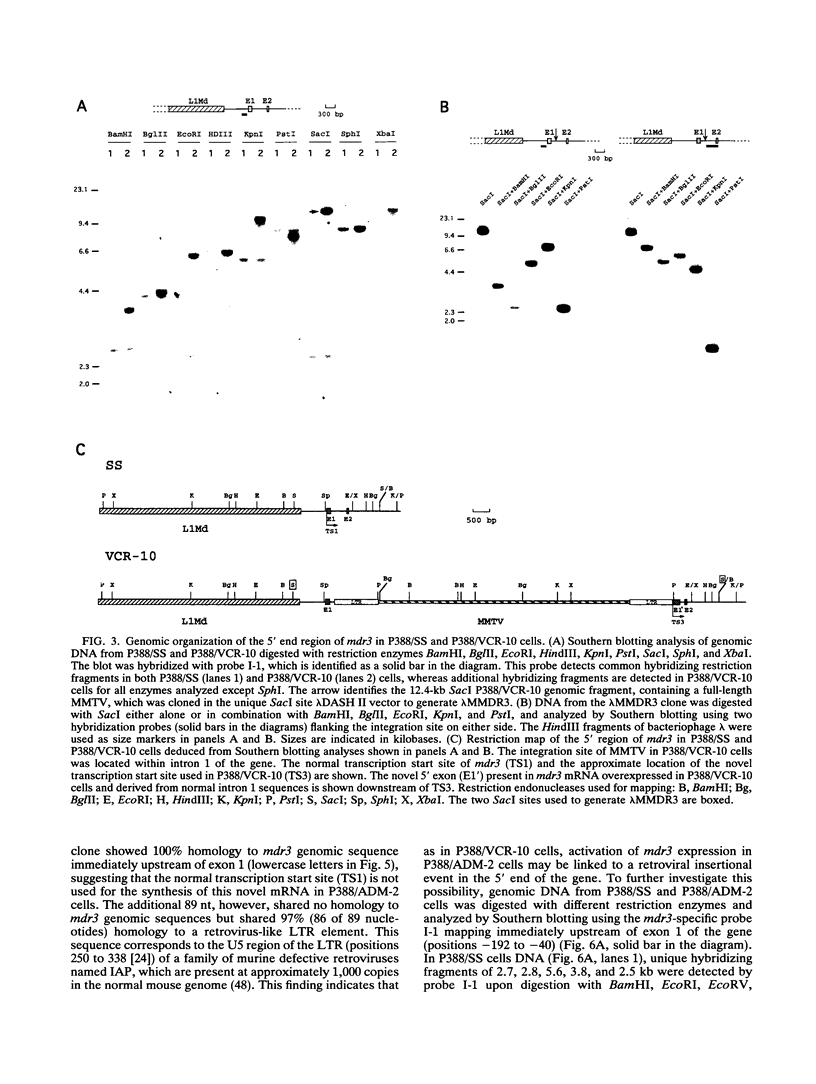
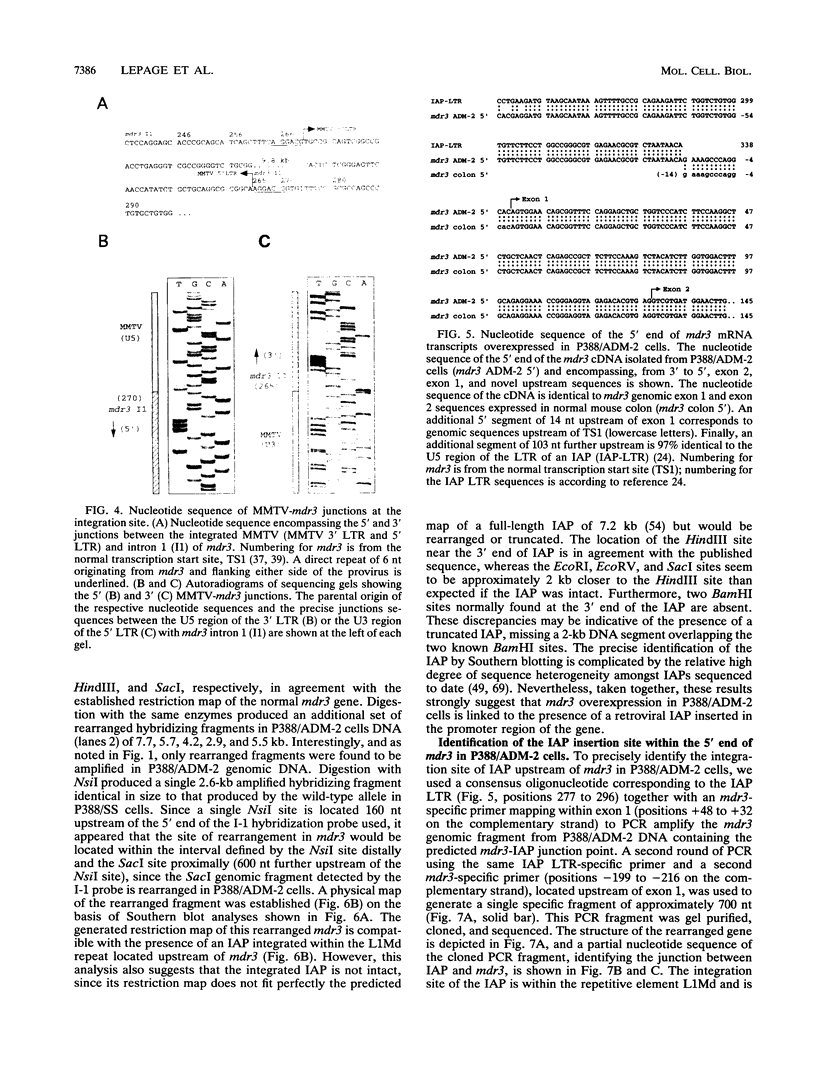
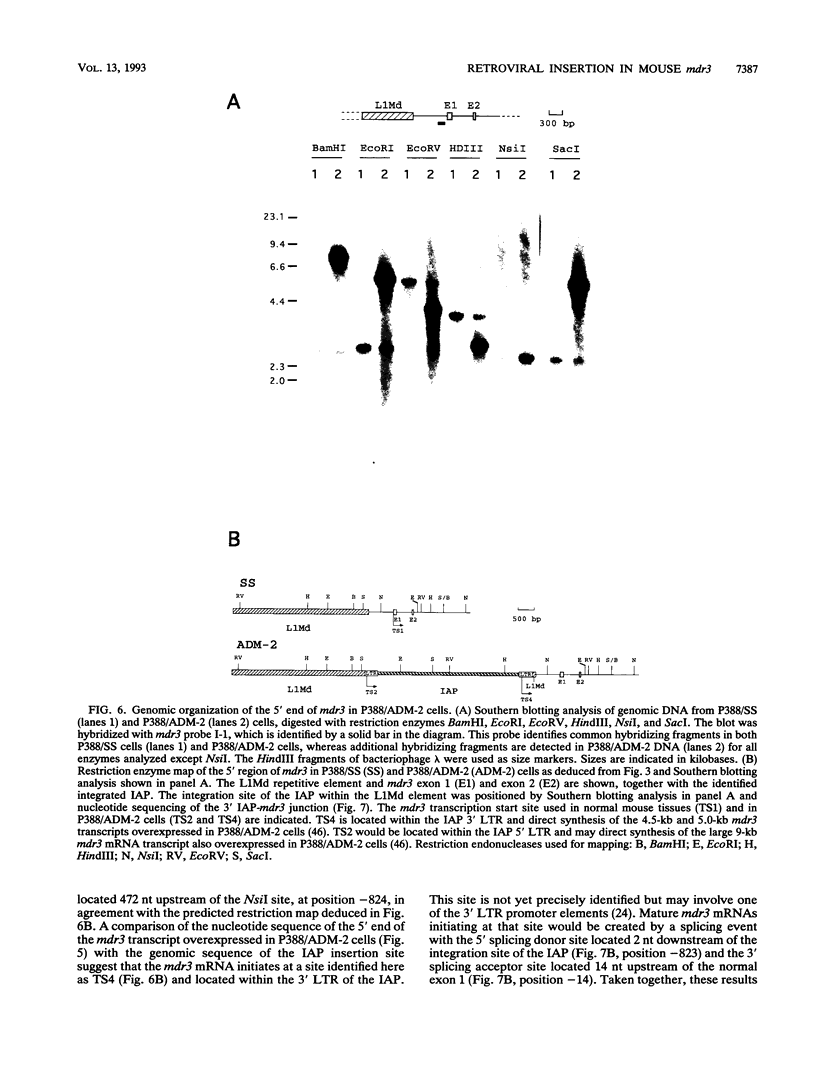
- Lepage P., Raymond M., Nepveu A., Gros P. Transcriptional activation of the mouse mdr3 gene coincides with the appearance of novel transcription initiation sites in multidrug-resistant P388 tumor cells. Cancer Res. 1993 Apr 1;53(7):1657–1664. [PubMed] [Google Scholar]
- Loeb D. D., Padgett R. W., Hardies S. C., Shehee W. R., Comer M. B., Edgell M. H., Hutchison C. A., 3rd The sequence of a large L1Md element reveals a tandemly repeated 5' end and several features found in retrotransposons. Mol Cell Biol. 1986 Jan;6(1):168–182. doi: 10.1128/mcb.6.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Sequences associated with intracisternal A particles are reiterated in the mouse genome. Cell. 1977 Dec;12(4):963–972. doi: 10.1016/0092-8674(77)90161-1. [DOI] [PubMed] [Google Scholar]
- Lueders K. K., Mietz J. A. Structural analysis of type II variants within the mouse intracisternal A-particle sequence family. Nucleic Acids Res. 1986 Feb 11;14(3):1495–1510. doi: 10.1093/nar/14.3.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden M. J., Morrow C. S., Nakagawa M., Goldsmith M. E., Fairchild C. R., Cowan K. H. Identification of 5' and 3' sequences involved in the regulation of transcription of the human mdr1 gene in vivo. J Biol Chem. 1993 Apr 15;268(11):8290–8297. [PubMed] [Google Scholar]
- Majors J. E., Varmus H. E. Nucleotide sequences at host-proviral junctions for mouse mammary tumour virus. Nature. 1981 Jan 22;289(5795):253–258. doi: 10.1038/289253a0. [DOI] [PubMed] [Google Scholar]
- Marchuk D., Drumm M., Saulino A., Collins F. S. Construction of T-vectors, a rapid and general system for direct cloning of unmodified PCR products. Nucleic Acids Res. 1991 Mar 11;19(5):1154–1154. doi: 10.1093/nar/19.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickley L. A., Bates S. E., Richert N. D., Currier S., Tanaka S., Foss F., Rosen N., Fojo A. T. Modulation of the expression of a multidrug resistance gene (mdr-1/P-glycoprotein) by differentiating agents. J Biol Chem. 1989 Oct 25;264(30):18031–18040. [PubMed] [Google Scholar]
- Mietz J. A., Grossman Z., Lueders K. K., Kuff E. L. Nucleotide sequence of a complete mouse intracisternal A-particle genome: relationship to known aspects of particle assembly and function. J Virol. 1987 Oct;61(10):3020–3029. doi: 10.1128/jvi.61.10.3020-3029.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R., Dixon M., Smith R., Peters G., Dickson C. Complete nucleotide sequence of a milk-transmitted mouse mammary tumor virus: two frameshift suppression events are required for translation of gag and pol. J Virol. 1987 Feb;61(2):480–490. doi: 10.1128/jvi.61.2.480-490.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R. Insertional mutagenesis in mouse mammary tumorigenesis. Curr Top Microbiol Immunol. 1991;171:43–65. doi: 10.1007/978-3-642-76524-7_3. [DOI] [PubMed] [Google Scholar]
- Nusse R., Theunissen H., Wagenaar E., Rijsewijk F., Gennissen A., Otte A., Schuuring E., van Ooyen A. The Wnt-1 (int-1) oncogene promoter and its mechanism of activation by insertion of proviral DNA of the mouse mammary tumor virus. Mol Cell Biol. 1990 Aug;10(8):4170–4179. doi: 10.1128/mcb.10.8.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R., van Ooyen A., Cox D., Fung Y. K., Varmus H. Mode of proviral activation of a putative mammary oncogene (int-1) on mouse chromosome 15. Nature. 1984 Jan 12;307(5947):131–136. doi: 10.1038/307131a0. [DOI] [PubMed] [Google Scholar]
- Peters G., Brookes S., Smith R., Placzek M., Dickson C. The mouse homolog of the hst/k-FGF gene is adjacent to int-2 and is activated by proviral insertion in some virally induced mammary tumors. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5678–5682. doi: 10.1073/pnas.86.15.5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekarz R. L., Cohen D., Horwitz S. B. Progesterone regulates the murine multidrug resistance mdr1b gene. J Biol Chem. 1993 Apr 15;268(11):7613–7616. [PubMed] [Google Scholar]
- Raymond M., Gros P. Cell-specific activity of cis-acting regulatory elements in the promoter of the mouse multidrug resistance gene mdr1. Mol Cell Biol. 1990 Nov;10(11):6036–6040. doi: 10.1128/mcb.10.11.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond M., Rose E., Housman D. E., Gros P. Physical mapping, amplification, and overexpression of the mouse mdr gene family in multidrug-resistant cells. Mol Cell Biol. 1990 Apr;10(4):1642–1651. doi: 10.1128/mcb.10.4.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J., Blondel B. J., Gallahan D., Callahan R. Mouse mammary tumor gene int-3: a member of the notch gene family transforms mammary epithelial cells. J Virol. 1992 Apr;66(4):2594–2599. doi: 10.1128/jvi.66.4.2594-2599.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelink H., Wagenaar E., Lopes da Silva S., Nusse R. Wnt-3, a gene activated by proviral insertion in mouse mammary tumors, is homologous to int-1/Wnt-1 and is normally expressed in mouse embryos and adult brain. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4519–4523. doi: 10.1073/pnas.87.12.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg M. L., Mickley L. A., Cole D. E., Balis F. M., Tsuruo T., Poplack D. G., Fojo A. T. Expression of the mdr-1/P-170 gene in patients with acute lymphoblastic leukemia. Blood. 1989 Sep;74(4):1388–1395. [PubMed] [Google Scholar]
- Schinkel A. H., Roelofs E. M., Borst P. Characterization of the human MDR3 P-glycoprotein and its recognition by P-glycoprotein-specific monoclonal antibodies. Cancer Res. 1991 May 15;51(10):2628–2635. [PubMed] [Google Scholar]
- Shen-Ong G. L., Cole M. D. Differing populations of intracisternal A-particle genes in myeloma tumors and mouse subspecies. J Virol. 1982 May;42(2):411–421. doi: 10.1128/jvi.42.2.411-421.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen D. W., Fojo A., Chin J. E., Roninson I. B., Richert N., Pastan I., Gottesman M. M. Human multidrug-resistant cell lines: increased mdr1 expression can precede gene amplification. Science. 1986 May 2;232(4750):643–645. doi: 10.1126/science.3457471. [DOI] [PubMed] [Google Scholar]
- Takatori T., Ogura M., Tsuruo T. Purification and characterization of NF-R2 that regulates the expression of the human multidrug resistance (MDR1) gene. Jpn J Cancer Res. 1993 Mar;84(3):298–303. doi: 10.1111/j.1349-7006.1993.tb02870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeter L. D., Becker F. F., Chisari F. V., Li D. J., Kuo M. T. Overexpression of the multidrug resistance gene mdr3 in spontaneous and chemically induced mouse hepatocellular carcinomas. Mol Cell Biol. 1990 Nov;10(11):5728–5735. doi: 10.1128/mcb.10.11.5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeter L. D., Eckersberg T., Tsai Y., Kuo M. T. Analysis of the Chinese hamster P-glycoprotein/multidrug resistance gene pgp1 reveals that the AP-1 site is essential for full promoter activity. Cell Growth Differ. 1991 Sep;2(9):429–437. [PubMed] [Google Scholar]
- Ueda K., Cardarelli C., Gottesman M. M., Pastan I. Expression of a full-length cDNA for the human "MDR1" gene confers resistance to colchicine, doxorubicin, and vinblastine. Proc Natl Acad Sci U S A. 1987 May;84(9):3004–3008. doi: 10.1073/pnas.84.9.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K., Clark D. P., Chen C. J., Roninson I. B., Gottesman M. M., Pastan I. The human multidrug resistance (mdr1) gene. cDNA cloning and transcription initiation. J Biol Chem. 1987 Jan 15;262(2):505–508. [PubMed] [Google Scholar]
- Ueda K., Pastan I., Gottesman M. M. Isolation and sequence of the promoter region of the human multidrug-resistance (P-glycoprotein) gene. J Biol Chem. 1987 Dec 25;262(36):17432–17436. [PubMed] [Google Scholar]
- Yanagawa S., Kakimi K., Tanaka H., Murakami A., Nakagawa Y., Kubo Y., Yamada Y., Hiai H., Kuribayashi K., Masuda T. Mouse mammary tumor virus with rearranged long terminal repeats causes murine lymphomas. J Virol. 1993 Jan;67(1):112–118. doi: 10.1128/jvi.67.1.112-118.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ymer S., Tucker W. Q., Sanderson C. J., Hapel A. J., Campbell H. D., Young I. G. Constitutive synthesis of interleukin-3 by leukaemia cell line WEHI-3B is due to retroviral insertion near the gene. Nature. 1985 Sep 19;317(6034):255–258. doi: 10.1038/317255a0. [DOI] [PubMed] [Google Scholar]
- Yu L., Cohen D., Piekarz R. L., Horwitz S. B. Three distinct nuclear protein binding sites in the promoter of the murine multidrug resistance mdr1b gene. J Biol Chem. 1993 Apr 5;268(10):7520–7526. [PubMed] [Google Scholar]
- van der Bliek A. M., Kooiman P. M., Schneider C., Borst P. Sequence of mdr3 cDNA encoding a human P-glycoprotein. Gene. 1988 Nov 30;71(2):401–411. doi: 10.1016/0378-1119(88)90057-1. [DOI] [PubMed] [Google Scholar]