Fig 2.
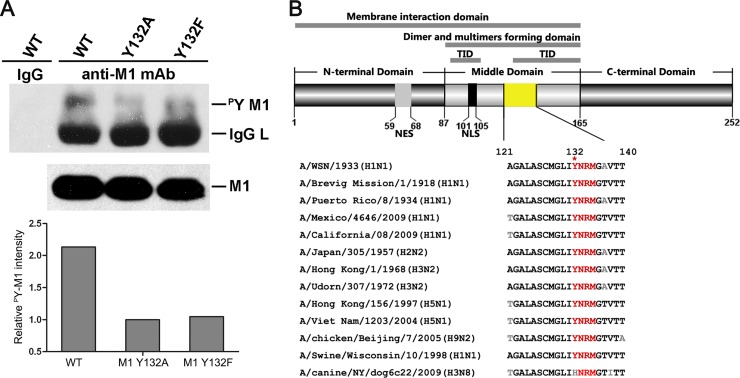
Tyrosine 132 is a key site of phosphotyrosine on M1. (A) Phosphotyrosine-containing M1 was detected by specific anti-p-Tyr antibodies in a virus rescue system, and 1/10 the amount of whole loading protein was probed with anti-M1 antibody to show the levels of total M1. The relative pY M1 intensity was determined as the ratio of tyrosine-phosphorylated M1 to total M1. The loss of phosphorylation at Y132 drastically reduced the level of total M1 tyrosine phosphorylation (lanes 3 and 4). (B) Y132 and the putative SH2 binding motif (YRNM) are greatly conserved in M1 proteins among different subtypes of influenza A virus. Sequence alignment of the specific M1 domain (yellow) was performed by MegAlign. The schematic diagram of M1 shows the functional domains involving Y132 (upper panel). WT, wild type; TID, transcription inhibition domain; NES, nuclear export signal; NLS, nuclear localization signal.