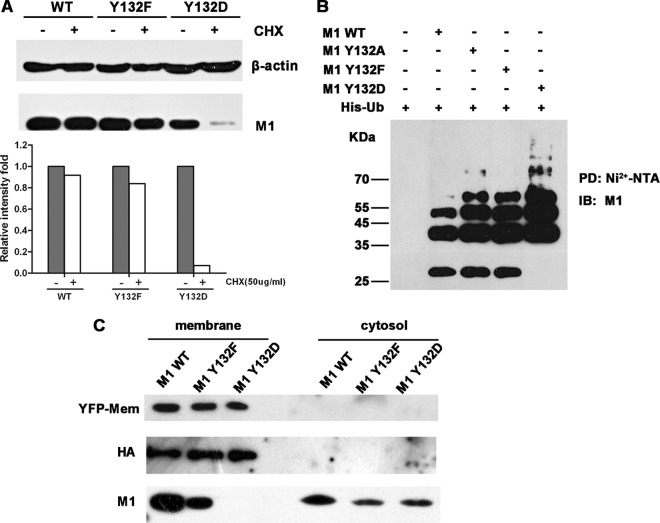
Fig 7.
Y-to-D substitution at site 132 impairs the intracellular stability and membrane association of M1. (A) Twenty-four hours after transfection with constructs for WT, Y132F, or Y132D M1, 293T cells were treated with or without CHX (50 μg/ml) for 4 h. Cells were lysed and then detected with the anti-M1 antibody; β-actin was used as an internal control. The relative intensities were calculated by quantifying the results shown in the upper panel. (B) An in vivo ubiquitination assay suggested that mutation of Y to D increased the ubiquitination of the M1 protein. 293T cells were transfected with expression constructs for Myc-tagged M1s and His-tagged ubiquitin (His-Ub). At 40 hours posttransfection, the proteasome inhibitor MG-132 (10 μM) was added to the cells for 4 h. Cell extracts were then immunoprecipitated with Ni2+-NTA beads. The eluted proteins were analyzed by Western blotting using an anti-M1 antibody. PD, pulldown. (C) 293T cells were transfected with 12 plasmids from the virus rescue system and with an expression construct for a membrane marker, pEYFP-Mem (BD). After a 72-h transfection period, samples of cellular membrane and cytoplasmic fractions were separated and detected by anti-yellow fluorescent protein (anti-YFP), anti-HA, and anti-M1 antibodies.