Fig 2.
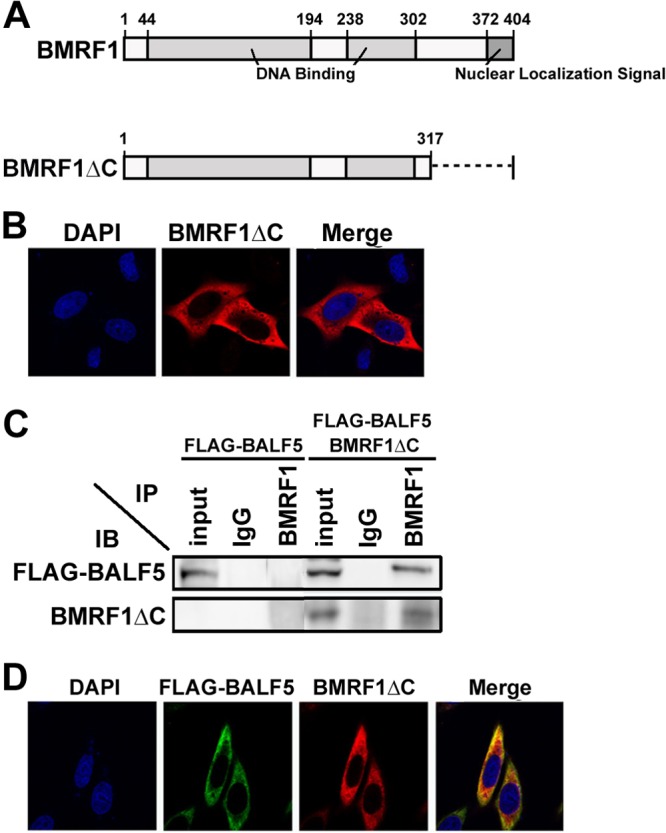
A BMRF1 mutant lacking the nuclear localization signal (NLS) does not support nuclear transport of the BALF5 DNA polymerase catalytic subunit. (A) Functional domains of BMRF1 protein. BMRF1 protein, consisting of 404 amino acids, has an NLS in its C terminus (amino acids 372 to 404). A BMRF1 mutant lacking the C-terminal 90 amino acids (aa 1 to 317; BMRF1ΔC) is indicated. (B) Subcellular localization of BMRF1ΔC. HeLa cells were transfected with pcDNA-BMRF1ΔC, fixed at 24 h posttransfection, immunostained with anti-BMRF1 (red), and examined by laser-scanning confocal microscopy. Nuclei were stained with DAPI (blue). (C) Physical interaction between BALF5 and BMRF1ΔC. HEK293T cells were transfected with pcDNA-FLAG-BALF5 alone or both pcDNA-FLAG-BALF5 and pcDNA-BMRF1ΔC. Cells were harvested at 24 h posttransfection, and immunoprecipitation (IP) analysis was carried out, as described in Materials and Methods. The antibodies used for IP and subsequent immunoblot (IB) analysis are indicated. (D) Subcellular localization of BALF5 in the presence of BMRF1ΔC. HeLa cells were transfected with pcDNA-FLAG-BALF5 and pcDNA-BMRF1ΔC, fixed at 24 h posttransfection, immunostained with anti-FLAG (green) and anti-BMRF1 (red), and examined by laser-scanning confocal microscopy. Nuclei were stained with DAPI (blue). The right panels are merged images.
