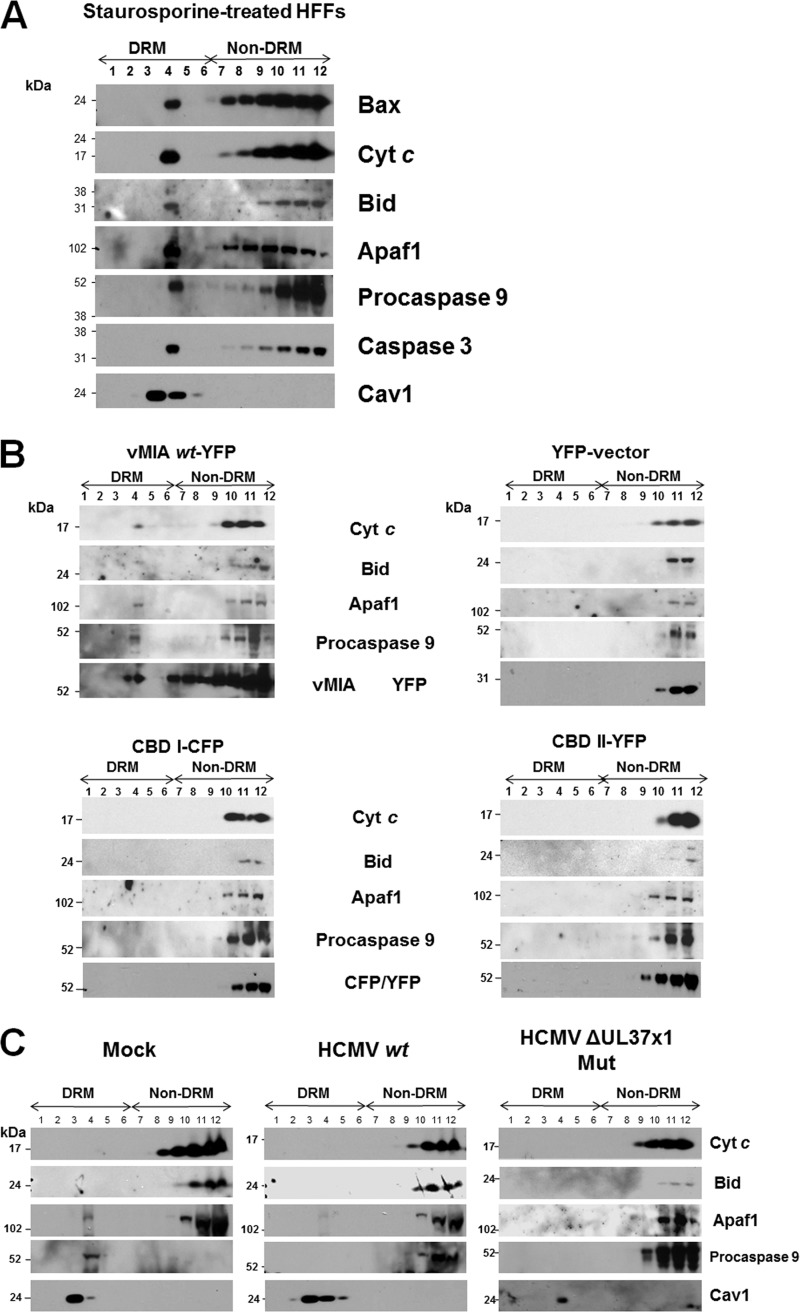
Fig 7.
(A) Association of Bax and apoptosome components and effectors with DRMs following STS treatment of HFFs. HFFs were treated with STS (50 nM) for 20 h. DRMs were isolated from the STS-treated cells and banded on flotation gradients as described previously (41). Aliquots of each fraction (30 μl) were separated by SDS-PAGE and examined for the presence of Bax (1:500; Millipore), Cyt c (1:200; Abcam), Bid (1:100; Santa Cruz), Apaf1 (1:100; Santa Cruz), procaspase 9 (1:500; GeneTex), caspase 3 (1:500; Millipore), and Cav1 (1:500; BD Biosciences) antibodies. (B) Lipid raft association of downstream apoptosome components requires vMIA lipid raft association. HFFs (1 × 107 cells) were transfected with vectors expressing pUL37x11–163-YFP, CBD I1–163-CFP, or CBDII1–163-YFP (41) and control YFP alone as in Fig. 6. Each gradient fraction (30 μl) was resolved by SDS-PAGE and examined using antibodies to Cyt c (1:200; Abcam), Bid (1:100; Santa Cruz), Apaf1 (1:100; Santa Cruz), procaspase 9 (1:500; GeneTex), and vMIA (DC35, 1:2,500) or mouse anti-GFP/YFP/CFP antibodies (1:200; Santa Cruz). Control anti-Cav1 is shown in Fig. 6. (C) HCMV infection inhibits recruitment of apoptosome components to lipid rafts. HFFs were mock infected or infected with HCMV wt (BADwt) or the HCMV ΔUL37x1 Mut (BADsubUL37x1) at an MOI of 1.5. Lipid rafts were isolated at 72 hpi, and gradient fractions (30 μl) were resolved by SDS-PAGE. Western blots performed using antibodies to Cyt c (1:200; Abcam), Bid (1:100; Santa Cruz), Apaf1 (1:100; Santa Cruz), procaspase 9 (1:500; GeneTex), and Cav1 (1:500; BD Biosciences).