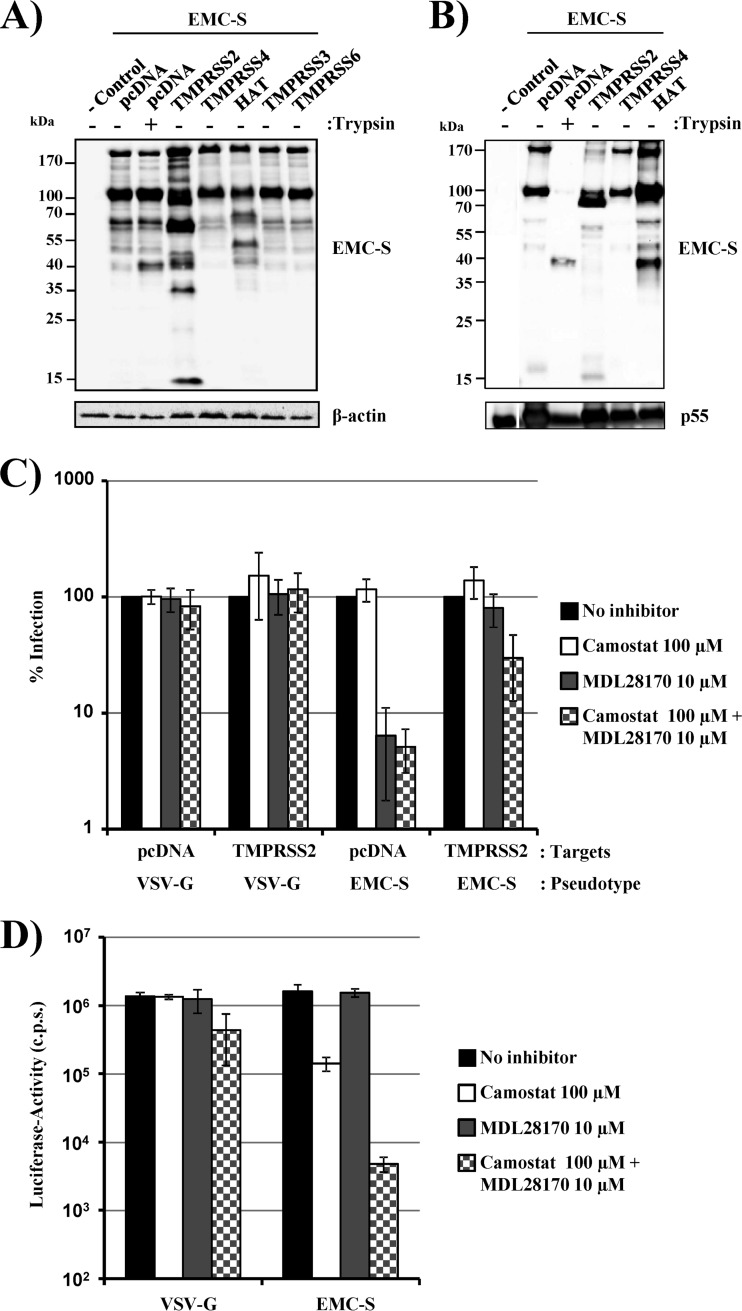
Fig 5.
TMPRSS2 activates EMC-S for cathepsin B/L-independent entry into target cells. (A) 293T cells were transfected with empty plasmid (−control) or cotransfected with plasmids encoding EMC-S and the indicated proteases. Forty-eight hours after transfection, cells were either left untreated or treated with trypsin, and lysates were analyzed by Western blotting using a V5 antibody or a β-actin antibody as a loading control. The results are representative of three independent experiments with different plasmid preparations. (B) Incorporation of cleaved EMC-S forms into lentiviral particles was assessed by coexpression of EMC-S (with a C-terminal V5 tag) with the indicated proteases and HIV-1 Gag. Virus-like particles were concentrated from the culture supernatants and analyzed by Western blotting using a V5 antibody, with a p55-Gag antibody used as a loading control. The experiment shown is representative of three independent experiments. (C) 293T cells were transiently transfected with empty plasmid (pcDNA) or a plasmid encoding TMPRSS2 and used as target cells for transduction by lentiviral vectors bearing EMC-S or VSV-G (as a control). Prior to infection, target cells were incubated with the indicated protease inhibitors. Luciferase activities in cell lysates were measured at 72 h postinfection. The average of three experiments (two for VSV-G-mediated transduction of TMPRSS2-expressing cells) performed in triplicate is shown. Transduction of untreated cells was set as 100%. Error bars indicate standard errors of the means (SEM). (D) Caco-2 cells, which express endogenous TMPRSS2, were incubated with DMSO or the indicated protease inhibitors, followed by transduction with lentiviral pseudotypes bearing EMC-S or VSV-G (as a control). At 72 h postinfection, luciferase activities in cell lysates were measured. The results of a representative experiment performed in triplicate are shown; error bars indicate SD. Comparable results were observed in two independent experiments.