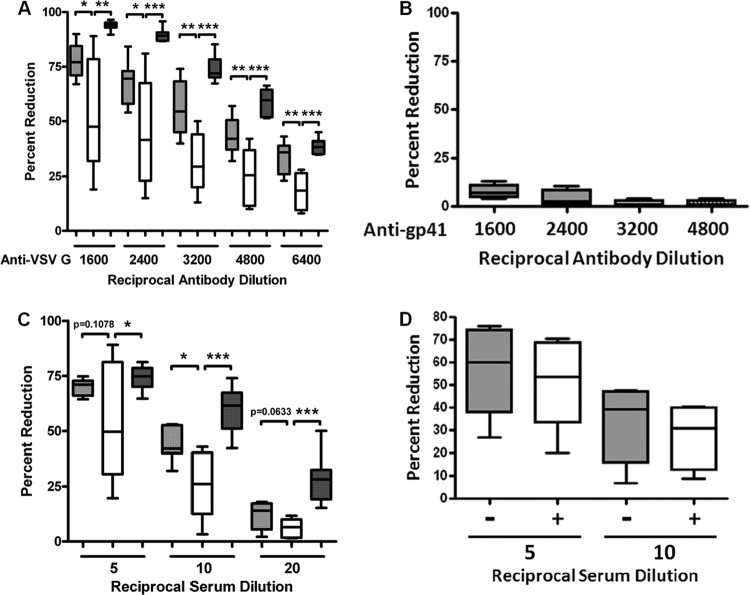
Fig 5.
Serum complement from HCV-infected patients impairs VSV infectivity. VSV was incubated with normal human sera, HCV-infected patient sera, or non-HCV liver disease patient sera in the presence or absence of VSV G-specific antibody, and the effect is shown as percent reduction of virus infectivity. Six individual sera were used for each set of experiments. (A) VSV was incubated with serial dilutions of antibody to VSV G prior to the addition of a 1:20 (5%) final dilution of HCV-specific patient sera (white bars), non-HCV liver disease patient sera (dark gray bars), or sera from healthy individuals (light gray bars). (B) Lack of complement-mediated neutralization of VSV reacted with non-VSV-specific antibody prior to a 1:20 dilution of human serum. Anti-gp41 MAb Chessie 8 (epitope PDRPEG) was used for this experiment. (C) Percent reductions of VSV infectivity were compared between these three groups of human sera after incubation with serial dilutions of HCV patient sera (light gray bars), non-HCV liver disease patient sera (dark gray bars), or sera from healthy individuals (white bars). (D) The classical pathway blocker, Mg-EGTA (final concentration of 10 mM MgCl2 and 10 mM EGTA), was added to HCV-positive patient serum, and the mixture was preincubated for 30 min at 37°C. A fixed number of VSV in duplicate samples were incubated with Mg-EGTA-treated HCV-positive sera at a 1:5 or 1:10 final dilution. Gray and white bars indicate Mg-EGTA-treated and Mg-EGTA-untreated HCV patient sera, respectively. (A and C) *, P < 0.05; **, P < 0.01; ***, P < 0.001 (compared to control results).