Abstract
Whole-body bioimaging was used to study dissemination of vaccinia virus (VACV) in normal and in immune deficient (nu−/nu−) mice protected from lethality by postchallenge administration of ST-246. Total fluxes were recorded in the liver, spleen, lungs, and nasal cavities of live mice after intranasal infection with a recombinant IHD-J-Luc VACV expressing luciferase. Areas under the flux curve were calculated for individual mice to assess viral loads. Treatment for 2 to 5 days of normal BALB/c mice with ST-246 at 100 mg/kg starting 24 h postchallenge conferred 100% protection and reduced viral loads in four organs compared to control mice. Mice also survived after 5 days of treatment with ST-246 at 30 mg/kg, and yet the viral loads and poxes were higher in these mice compared to 100-mg/kg treatment group. Nude mice were not protected by ST-246 alone or by 10 million adoptively transferred T cells. In contrast, nude mice that received T cells and 7-day treatment with ST-246 survived infection and exhibited reduced viral loads compared to nonreconstituted and ST-246-treated mice after ST-246 was stopped. Similar protection of nude mice was achieved using adoptively transferred 1.0 and 0.1 million, but not 0.01 million, purified T cells or CD4+ or CD8+ T cells in conjunction with ST-246 treatment. These data suggest that ST-246 protects immunocompetent mice from lethality and reduces viral dissemination in internal organs and poxvirus lesions. Furthermore, immune-deficient animals with partial T cell reconstitution can control virus replication after a course of ST-246 and survive lethal vaccinia virus challenge.
INTRODUCTION
Vaccination of the general public against smallpox was discontinued in the United States in 1972 after the massive worldwide vaccination campaign that eradicated smallpox. However, the threat of the potential release of variola virus (the causative agent of smallpox) as a bioterrorist agent and the emergence of monkeypox virus infections in humans led to a renewed interest in the development of antiviral drugs and safer vaccines (1–3). The effectiveness of preventive smallpox vaccination with the Dryvax vaccine (that has been replaced with a newly licensed vaccine ACAM2000) has been well documented (4, 5). However, little is known about the protective capacity of smallpox vaccines when used after exposure to variola virus. Earlier studies have concluded that in the event of an outbreak, there is a very small window of about 4 days when vaccine can be administered in order to prevent or significantly ameliorate subsequent illness and provide protection from a fatal outcome (reviewed in reference 1). The currently licensed smallpox vaccine containing replicating vaccinia virus (VACV) poses a significant risk to individuals with certain skin disorders or immunodeficiency conditions (6, 7). To manage cases of vaccine-associated complications and for treatments of accidental exposures, several new antiviral compounds are being investigated in animal models and in clinical trials in humans (8).
Currently, only cidofovir (CDV), a nucleoside analog approved for the treatment of cytomegalovirus retinitis in AIDS patients, is permitted for use as emergency treatment in the case of a smallpox outbreak (9). Several limitations of CDV, such as the requirement for intravenous dosing and high incidence of acute kidney toxicity, promoted the development of CMX001, a lipid conjugate of CDV, which is orally bioavailable and does not induce nephrotoxicity. CMX001 is being evaluated in animal models according to the U.S. Food and Drug Administration (FDA) Animal Rule for licensure of new smallpox treatments (10).
ST-246 is a low-molecular-weight compound identified through a high-throughput screening of >300,000 compounds for their ability to inhibit VACV-induced cytopathic effects in Vero cells (11). In vitro, ST-246 was found to be active against multiple species of orthopoxviruses, including VACV, cowpox virus, ectromelia virus, monkeypox virus, camelpox virus, and variola virus, with a 50% effective concentration of ≤0.07 μM (11). ST-246 exerts its antiviral activities by targeting F13L (p37), a specific viral phospholipase that plays an important role in egress of viral particles and production of extracellular virus (11). In vivo, the daily administration of ST-246 for 5 to 14 days has been shown to protect normal mice from lethal intranasal challenge with ectromelia and cowpox viruses (11, 12). In immune-deficient mice, protection was observed in mice receiving treatment; however, the mice succumbed after ST-246 treatment ceased (13). Importantly, in nonhuman primates, ST-246 has been shown to protect from poxvirus disease, reduce viral loads, and enhance survival (14). Clinical trials showed that ST-246 was well tolerated by human subjects after daily oral administration (15). Moreover, ST-246, together with VIGIV and CMX001, was recently successfully used for the treatment of progressive vaccinia, demonstrating the advantage of combination therapy utilizing drugs that target different stages of VACV infection (16).
Previous publications and studies in our laboratory have shown that whole-body bioimaging provides an advantage over more traditional methods for assessing the effectiveness of vaccines and immunoglobulin-based treatments using mouse models of respiratory VACV infection (17–20). In the present study, we used bioimaging and statistical analysis of viral loads calculated as the area under the flux curve (AUC) to further define mechanisms of protection from lethal challenge conferred by ST-246 in normal and in immune-deficient mice. Using this approach, we explored the impact of drug dose and of time of treatment initiation and termination on mortality, viral loads, as well as dermal pox development, in normal mice. Previous studies have shown that ST-246 extended survival but did not rescue T-cell-deficient nude mice from lethality, suggesting that B cells alone are not sufficient for protection in the presence of ST-246 treatment. In the present study, we sought to determine whether under the same conditions, transferred T cells would protect nude mice from lethality. We showed that adoptively transferred T cells (or T cell subsets) in combination with ST-246 therapy not only protected nude mice from lethal challenge but also prevented viral rebound after the discontinuation of ST-246 treatment and protected animals from rechallenge without the need for additional ST-246 treatment.
MATERIALS AND METHODS
Virus.
A recombinant VACV expressing luciferase (IHD-J-Luc) was constructed based on the International Health Department J (IHD-J) strain of VACV. The luciferase gene (under the control of the synthetic E/L promoter) was inserted at a truncated host range gene locus equivalent to the cowpox gene, CPX077. Details of the construction and characterization of sucrose gradient purified IHD-J-Luc were previously described (19, 20; C. Meseda et al., unpublished data). BSC-1 cells were infected with IHDJ-Luc, and the titer of viral stock was determined using BSC-40 cells. The plaque phenotype of IHD-J-Luc is similar to wild-type IHD-J VACV in plaque size and in the formation of comet-like plaques under liquid medium overlay (C. Meseda, unpublished data). The IHD-J strain infects and replicates in NIH 3T3 mouse cells (12). A single stock of IHD-J-Luc VACV containing 3.9 × 109 PFU/ml was used throughout the study.
Preparation of cells for in vivo adoptive transfer.
T cells, CD4+ T cells, and CD8+ T cells were obtained from the spleens of normal female BALB/c mice at 6 to 8 weeks of age (National Cancer Institute, Frederick, MD) using mouse pan T cell, mouse CD4+ T cell, and mouse CD8α+ T cell isolation kits II (Miltenyi Biotec, Inc., Auburn, CA), respectively, according to the manufacturer's instructions. The obtained subsets were 99% pure as verified by flow cytometry.
Mice and protocols for in vivo treatments.
Five-week-old female BALB/c mice or 7- to 8-week-old BALB/c nude mice (National Cancer Institute) were used in all experiments. Immediately prior to challenge, mice were anesthetized using 2,2,2-tribromoethanol dissolved in tertiary amyl alcohol and diluted in sterile phosphate-buffered saline (PBS) according to the manufacturer's instructions. The anesthesia was administered at 20 μl per g of body weight by intraperitoneal injection. Mice were challenged via the intranasal route with 105 PFU (10 50% lethal doses [LD50]) or 104 PFU (1 LD50) of IHD-J-Luc for normal and for nu/nu mice, respectively, in a 10-μl volume delivered in one nostril.
For postchallenge treatments, ST-246 at 100 mg/kg (or as specified in the study) or vehicle control were administered in 100-μl volumes via oral gavage using 38.1-mm Animal Feeding Needles (Cadence Science, Inc., Cranston, RI) starting at 24 h postinfection or as specified.
For the adoptive-transfer experiment, purified T cells, or CD4+ or CD8+ T cell subsets from normal BALB/c mice in 400-μl volumes in PBS were injected into the tail veins of nude mice 24 h before challenge using 1-ml insulin syringes with 28G 0.5-in. needles (Becton Dickinson). The handling of mice and experimental procedures were approved by the Center for Biologics Evaluation and Research animal study review committee. Group sample sizes for each experiment are specified in the figure legends.
In vivo measurements of luciferase activity.
The details of whole-body imaging using IVIS 50 instrument (Caliper Life Sciences, Hopkinton, MA) were previously described (21). In brief, mice received a single 150-μg/g (body weight) intraperitoneal injection of d-luciferin (potassium salt) (Caliper) 10 to 15 min prior to imaging. Mice were anesthetized in an oxygen-rich induction chamber with 2% isoflurane and were imaged daily on days 1 to 10 postchallenge or as specified. In some experiments, mice were sacrificed immediately after imaging, and the ovaries were excised, placed into the wells of 24-well plates in RPMI medium, and subjected to imaging. Images were analyzed with the LivingImage 3.02 software according to the manufacturer's instructions (Caliper). The background bioluminescence was determined using images of d-luciferin-injected animals 1 day prior to infections. In all calculations, the background bioluminescence was subtracted from experimental values. Poxvirus lesions were counted on mouse tails using dorsal images as previously described (22).
Statistical analysis.
Kaplan-Meier survival curves of time-to-death after infection were generated using GraphPad Prism v5 software. The AUC was calculated for each mouse; mean AUCs of various groups were compared using two-sample t tests. For each set of comparisons, the flux curves were truncated at the last time point at which all mice had complete data, to ensure fair comparisons between groups. We also used two-sample t test to compare the number of poxes between groups. All results were considered statistically significant at a P of ≤0.05 (two-tailed). We performed these analyses using version 2.15 of the R statistical software package (23).
RESULTS
Effect of the length of ST-246 treatment on animal survival and on IHD-J-Luc viral loads in the organs of normal mice.
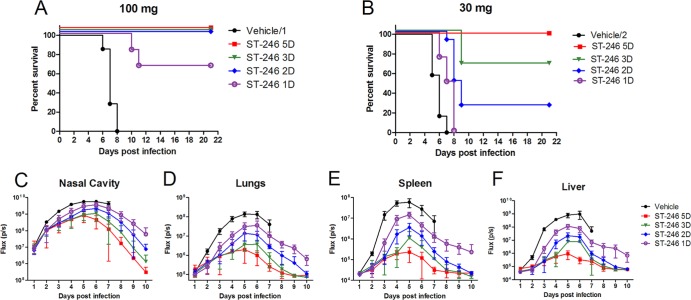
Previous studies showed that orally administered ST-246 twice per day for 14 days protected immunocompetent mice from lethal orthopoxvirus challenge (11). To determine the minimal duration of treatment with ST-246 that confers protection from lethality, BALB/c mice were infected with 105 PFU of IHD-J-Luc virus via the intranasal route, and were treated daily for 1, 2, 3, or 5 days with either a full dose of ST-246 (100 mg/kg) in experiment 1 or with a suboptimal dose (30 mg/kg) in experiment 2 (6 animals/group), or with vehicle control, starting at 24 h postinfection (Fig. 1). To monitor virus dissemination from the site of infection to the lungs and internal organs, mice were subjected to whole-body bioimaging daily for 10 days using the IVIS 50 instrument. All mice that received vehicle control succumbed within 8 days postchallenge (Fig. 1A and B, black circles). Treatment with a full dose of ST-246 (100 mg/kg) for 2, 3, or 5 days protected 100% of mice, while 1 day of treatment resulted in 66% survival (Fig. 1A, purple circles). At the suboptimal dose (30 mg/kg), only the 5-day treatment protected 100% of animals. Treatment for 2 to 3 days provided partial protection, and mice that received a single injection of ST-246 at 30 mg/kg did not survive (Fig. 1B).
Fig 1.
Two days of treatment with ST-246 at 100 mg/kg dose starting 24 h postchallenge with IHD-J-Luc VACV was required to protect 100% of BALB/c mice from lethality. (A to F) Lethality outcome and bioluminescence measurements in the internal organs of BALB/c mice infected with recombinant IHD-J-Luc VACV. BALB/c mice were infected intranasally with IHD-J-Luc VACV at 105 PFU. Starting 24 h postchallenge, mice received daily treatments with vehicle alone for 5 days (black circles) or with ST-246 for 1 day (purple open circle), 2 days (blue diamond), 3 days (green triangle), or for 5 days (red square) (A to F) at 100 mg/kg (A, C to F) or at 30 mg/kg (B). Mice were observed for survival (A and B) and were subjected to whole-body bioimaging (C to F). Total fluxes in the nasal cavity (B), lungs (D), liver (E), and spleen (F) were determined and used to calculate the mean total flux ± the standard deviation (SD) using a Student t test. Vehicle/1, 28 animals per group; vehicle/2, 18 animals per group; all ST-246-treated mice, 6 mice per group.
Images of infected mice were processed using LivingImage software to quantify total photon fluxes emitted by infected organs in individual mice, and mean total fluxes were calculated as previously described (20). During the first 4 days postinfection, mean total fluxes increased in all groups of mice in all organs and reached maximum on days 4 to 5, after which they gradually declined in all animals that survived (Fig. 1C to F). Mean total fluxes detected in all four organs were highest in mice that received vehicle (black circles) and lowest in mice that were treated for 5 days with full dose (red squares) or with suboptimal dose of ST-246 (Fig. 1C to F and data not shown). By day 10, bioluminescence in internal organs returned to background levels in animals that received ST-246 for 3 or 5 days, and the rest of survived animals cleared infection in all organs by the end of the observation period (day 21 [data not shown]).
To determine whether ST-246 significantly reduced viral loads in the internal organs, AUC values were calculated for individual mice for days 1 through 5 when all animals were alive in experiments 1 and 2. Mean AUCs were compared between ST-246-treated and vehicle-treated animals (Table 1). For days 1 to 5, AUCs in all organs were significantly lower in mice that received ST-246 at 100 mg/kg compared to vehicle control mice (vehicle/1). In mice that received 30 mg of ST-246/kg, the AUCs for days 1 to 5 were significantly lower in the lungs, spleen, and liver but not in the nasal cavity compared to vehicle control (vehicle/2). To further investigate how the dose of ST-246 treatment affected viral loads, mean AUCs calculated for each organ were compared between mice that received full and suboptimal dose of ST-246. A Student t test confirmed that the AUCs were higher in spleens and in livers of mice that received ST-246 at 30 mg/kg compared to the 100-mg/kg dose for 1, 2, 3, or 5 days. AUCs in the nasal cavity and in the lungs were significantly different between the 30-mg/kg and the 100-mg/kg groups in animals that were treated for 3 and 5 days and in nasal cavity or in the lungs in animals that were treated for 2 days or for 1 day, respectively (Table 1).
Table 1.
Mean AUCs for BALB/c mice infected with 105 PFU of IHD-J-Luc VACV and treated daily with ST-246 at 200 or 30 mg/kg postchallenge
| Treatment | Mean AUC (log10 p/s × day) ± 2×SEa |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nasal cavity |
Lungs |
Liver |
Spleen |
|||||||||
| ST-246 |
MD | ST-246 |
MD | ST-246 |
MD | ST-246 |
MD | |||||
| 100 mg/kg | 30 mg/kg | 100 mg/kg | 30 mg/kg | 100 mg/kg | 30 mg/kg | 100 mg/kg | 30 mg/kg | |||||
| Vehicle | 38.8 ± 0.4 | 39.5 ± 0.6 | 30.2 ± 0.4 | 30.9 ± 0.7 | 30.9 ± 0.4 | 31.9 ± 0.7 | 28.1 ± 0.4 | 28.7 ± 0.5 | ||||
| ST-246 (1 day) | 37.2 ± 0.8* | 38.4 ± 0.9 | 1.2 | 27.3 ± 0.8** | 29.0 ± 0.6** | 2.7* | 27.2 ± 0.4** | 29.7 ± 0.6** | 2.5** | 25.0 ± 0.6** | 26.7 ± 0.6** | 1.7* |
| ST-246 (2 days) | 36.7 ± 0.5** | 38.7 ± 0.6 | 2.0** | 26.7 ± 0.6** | 27.6 ± 0.9** | 0.9 | 24.9 ± 0.9** | 27.7 ± 0.8** | 2.8** | 23.2 ± 0.7** | 24.9 ± 0.7** | 1.7* |
| ST-246 (3 days) | 36.3 ± 1.3** | 39.3 ± 0.7 | 3.0* | 25.8 ± 1.0** | 27.7 ± 0.2** | 1.9** | 23.7 ± 1.0** | 27.6 ± 0.4** | 3.9** | 21.9±1.3** | 25.3 ± 0.4** | 3.4** |
| ST-246 (5 days) | 35.6 ± 2.2** | 38.6 ± 0.7 | 3.0* | 25.7 ± 0.5** | 28.2 ± 0.7** | 2.5* | 23.8 ± 0.5** | 27.0 ± 0.4** | 3.2** | 21.7 ± 0.4** | 24.6 ± 0.8** | 2.9** |
The mean AUC values were calculated for fluxes in mice for days 1 to 5. ST-246 was tested at 100 or 30 mg/kg, as indicated, and the mean difference (MD) for each pair of values is also indicated. Asterisks in the 100- and 30-mg/kg columns reflect significant differences in the mean AUC between groups of mice that received ST-246 versus vehicle/1 for the 100-mg/kg group or vehicle/2 for the 30-mg/kg group. Asterisks in the MD columns reflect significant differences in mean AUCs in mice that received similar durations of treatment with 100-mg/kg versus 30-mg/kg doses of ST-246.
, P ≤ 0.05;
, P ≤ 0.001 (two-sample t test). p/s, photons per second.
In addition to fluxes in organs, we used dorsal images of infected mice to count poxes on mouse tails (Table 2) (22). No poxes were detected in mice prior to day 3 postinfection (data not shown). In mice that received vehicle, a maximum numbers of poxes were detected on days 5 and 6 (average of 6.7 to 10.3 poxes/animal/day). Treatment with ST-246 at 100 mg/kg significantly reduced poxes in all mice compared to the control group between days 3 and 6. After the ST-246 treatment was stopped, poxes appeared in animals that were treated for <3 days, while very few poxes were detected in mice receiving 100 mg of ST-246/kg for 5 days. The same trend was observed in mice that received ST-246 at 30 mg/kg. However, there was a dose-dependent increase in pox counts: in 5-day treatment groups, higher numbers of poxes were detected after treatment termination (i.e., days 6 to 10) in mice that received 30 mg of ST-246/kg compared to those receiving 100 mg/kg (Table 2).
Table 2.
Pox counts in mice that received ST-246 at 100 or at 30 mg/kg for 1, 2, 3, or 5 days
| Treatment | Dose (mg/kg) | Avg pox no./animal/day ± SDa at day postinfection: |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| Expt 1 | |||||||||
| Vehicle (5 days) | 0.9 ± 0.3 | 5.9 ± 0.8 | 9.5 ± 0.9 | 10.3 ± 0.5 | NA | NA | NA | NA | |
| ST-246 (5 days) | 100 | 0** | 0** | 0.3 ± 0.5** | 0** | 0.3 ± 0.5 | 0.3 ± 0.5 | 0.2 ± 0.4 | 0.2 ± 0.4 |
| ST-246 (3 days) | 100 | 0** | 0** | 1.2 ± 1.3** | 3.2 ± 3.5** | 3.3 ± 2.5 | 3.0 ± 2.4 | 1.8 ± 1.6 | 1.3 ± 1.5 |
| ST-246 (2 days) | 100 | 0** | 0.5 ± 0.8** | 2.8 ± 2.1** | 5.2 ± 3.3* | 6.3 ± 2.9 | 7.2 ± 1.8 | 5.3 ± 3.1 | 3.0 ± 1.8 |
| ST-246 (1 day) | 100 | 0** | 1.2 ± 1.2* | 7.7 ± 1.8* | 8.5 ± 1.8* | 9.2 ± 3.1 | 10.2 ± 3.1 | 10.2 ± 2.6 | 7.4 ± 3.5 |
| Expt 2 | |||||||||
| Vehicle (5 days) | 2.0 ± 1.9 | 4.2 ± 2.1 | 7.2 ± 2.9 | 6.7 ± 2.9 | NA | NA | NA | NA | |
| ST-246 (5 days) | 30 | 0* | 1.3 ± 1.2* | 2.7 ± 1.9* | 3.8 ± 2.6 | 4.5 ± 2.4 | 4.7 ± 2.3 | 4.8 ± 3.1 | 4.0 ± 2.9 |
| ST-246 (3 days) | 30 | 0* | 0.8 ± 1.2* | 4.3 ± 1.4 | 5.5 ± 1.2 | 7.5 ± 2.1 | 8.3 ± 2.0 | 8.4 ± 2.2 | 7.8 ± 1.7 |
| ST-246 (2 days) | 30 | 0.3 ± 0.5 | 1.5 ± 1.5 | 4.8 ± 1.5 | 6.0 ± 1.5 | 7.7 ± 2.5 | 4.0 ± 0.0 | NA | NA |
| ST-246 (1 day) | 30 | 0.5 ± 0.8 | 2.7 ± 1.5 | 5.5 ± 2.3 | 8.3 ± 2.9 | 8.0 ± 2.2 | 7.0 ± 0.0 | NA | NA |
Poxes on mouse tails were counted using images of individual mice and were used to calculate average pox number per survived animal for each day. Asterisks denote significant differences between ST-246 and vehicle groups in mean daily pox count.
, P ≤ 0.05;
, P ≤ 0.001. NA, not applicable.
VACV is known to replicate to high titers in the ovaries of female mice (24). To determine whether ST-246 treatment had any effect on the viral dissemination to the ovaries, BALB/c mice were infected with IHD-J-Luc and treated with vehicle or with a full dose of ST-246 daily for 3 days (Fig. 2). On days 3 to 5 postinfection, bioluminescence signals were detected in the spleens and lungs in both groups of mice, but the intensity was clearly lower in the ST-246-treated animals (Fig. 2a, e, and i versus Fig. 2c, g, and k). Ex vivo bioimaging of isolated ovaries detected bioluminescence signals only in ovaries from vehicle-treated animals (Fig. 2b, f, and j) but not in ovaries from ST-246-treated animals (Fig. 2d, h, and l).
Fig 2.
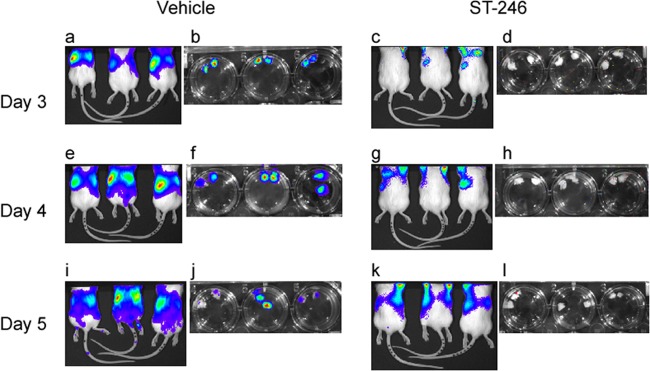
ST-246 treatment prevented dissemination of IHD-J-Luc VACV into the ovaries of normal BALB/c mice. BALB/c mice (9 mice per group) were infected with IHD-J-Luc and were treated daily for 3 days with vehicle (a, b, e, f, i, and j) or with a full dose of ST-246 (c, d, g, h, k, and l). On days 3 (a to d), 4 (e to h), and 5 (i to l), 3 mice per group were imaged and, immediately after imaging, mice were sacrificed, and the ovaries were imaged ex vivo in the wells of a 24-well plate. Dorsal images of live mice and of extracted ovaries (two ovaries from one mouse in a single well) are shown in panels a, c, e, g, i, and k and in panels b, d, f, h, j, and l, respectively.
These data demonstrated that at least 2 days of treatment with full dose of ST-246 (100 mg/kg) was required to protect immunocompetent mice from lethality and that postchallenge treatment with full dose of ST-246 for 5 days significantly reduced viral loads in multiple organs and in the skin (pox lesions). Treatment with a suboptimal dose (30 mg/kg) for 5 days protected animals from lethality but was less effective in curtailing virus replication in internal organs and in preventing pox formation compared to the 100-mg/kg treatment.
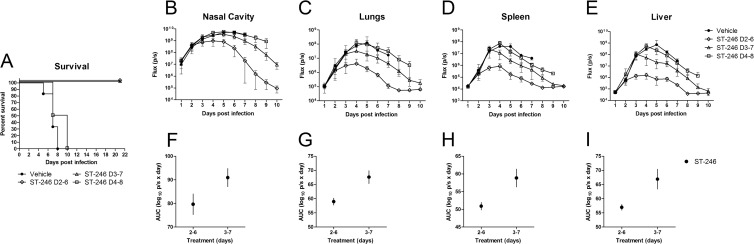
Treatment with ST-246 can be delayed for up to 3 days (but not 4 days) postinfection.
In the event of a smallpox outbreak, initiation of antiviral treatment may be delayed inadvertently for some exposed individuals. To investigate whether ST-246 administration can be delayed without compromising protection, BALB/c mice were infected with IHD-J-Luc VACV and treated for 5 days with vehicle alone or with ST-246 at 100 mg/kg starting on day 2, 3, or 4 postinfection (Fig. 3). Initiation of treatment on day 4 did not protect animals from lethality. The survival curve for this group appeared similar to that of the vehicle control group (Fig. 3A). In contrast, initiation of treatment on days 2 or 3 conferred full protection from lethality (Fig. 2A). Mice were subjected to bioimaging daily for 10 days (Fig. 3B to E). Fluxes recorded in vehicle-treated animals (closed circles) and in mice that started ST-246 treatment on day 4 (open squares) were very similar in all four organs, confirming the lack of control of virus replication and dissemination (Fig. 3B to E). In mice that received ST-246 on day 2 or 3 postinfection, an initial rise in fluxes was observed during first 2 or 3 days, which was followed by a gradual decline, coinciding with the initiation of treatment. All mice that received ST-246 with a 2- or 3-day delay survived. However, AUCs calculated through day 9 postinfection (when both groups completed treatment) were significantly lower in mice that started ST-246 treatment on day 2 compared to those that started treatment on day 3 (Fig. 3F to I).
Fig 3.
ST-246 started 2 or 3 days, but not 4 days, postinfection protected mice from lethal challenge with IHD-J-Luc VACV. BALB/c mice were infected with IHD-J-Luc as described in Fig. 1 and were treated for a total of 5 days with vehicle on days 2 to 6 (●) or with ST-246 at 100 mg/kg on days 2 to 6 (♢), days 3 to 7 (△), or days 4 to 8 (□) (A to E). Mice were observed for lethality (A) and were subjected to bioimaging daily for 10 days (B to E). Total fluxes in the nasal cavity (B), lungs (C), spleen (D), and liver (E) were determined and used to calculate the mean total flux ± the SD using a Student t test. (F to I) AUCs were calculated for fluxes from individual mice for days 1 to 10 in the nasal cavities (F), lungs (G), spleens (H), and livers (I) of mice that received ST-246 on day 2 to 6 and days 3 to 7 only. The data are shown as mean AUCs ± 2×SE for groups of 6 mice per group. The experiment was performed twice with similar results.
The same mice were used to monitor pox development (Table 3). In vehicle-treated mice, the number of poxes/animal peaked around days 6 to 7. As expected, the numbers of poxes were not significantly different between mice that received ST-246 treatment starting on day 4 and control mice. Importantly, a delay of 2 days, followed by a 5-day treatment with ST-246, prevented pox development, while a further delay by 1 day reduced, but did not prevent, pox development in some mice. Together, these data demonstrated that a delay in ST-246 treatment for up to 2 days provided complete protection from lethality, significantly reduced viral loads in internal organs, and prevented pox development in mice even after treatment termination.
Table 3.
Pox counts in BALB/c mice that received ST-246 at 100 mg/kg starting 2, 3, and 4 days postinfection
| Treatment | Avg pox no./animal/day ± SDa at day postinfection: |
|||||||
|---|---|---|---|---|---|---|---|---|
| 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Vehicle | 1.2 ± 1.6 | 5.5 ± 2.8 | 9.0 ± 2.4 | 12.0 ± 3.1 | 12.5 ± 0.7 | NA | NA | NA |
| ST-246 (days 2 to 6) | 0.2 ± 0.4 | 0.5 ± 0.8* | 0.8 ± 1.0** | 0.7 ± 1.2** | 0** | 0 | 0 | 0 |
| ST-246 (days 3 to 7) | 0.7 ± 0.8 | 2.5 ± 2.3 | 3.2 ± 2.6* | 2.7 ± 2.5** | 3.3 ± 2.7 | 2.5 ± 1.6 | 2.5 ± 2.3 | 2.5 ± 1.8 |
| ST-246 (days 4 to 8) | 1.0 ± 1.5 | 4.5 ± 3.7 | 6.2 ± 2.6 | 6.5 ± 3.5 | 5.7 ± 4.7 | 5.0 ± 3.6 | 4.0 ± 3.5 | NA |
Asterisks denote significant differences between ST-246 and vehicle groups in mean daily pox counts as follows: *, P ≤ 0.05; and **, P ≤ 0.001. NA, not applicable.
Protection of nude mice from lethality and viral dissemination requires adoptively transferred T cells prior to ST-246 treatment.
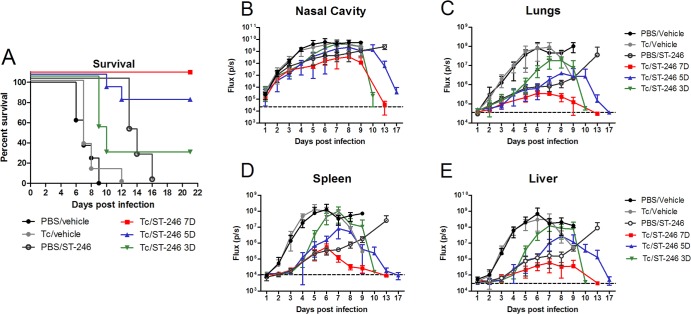
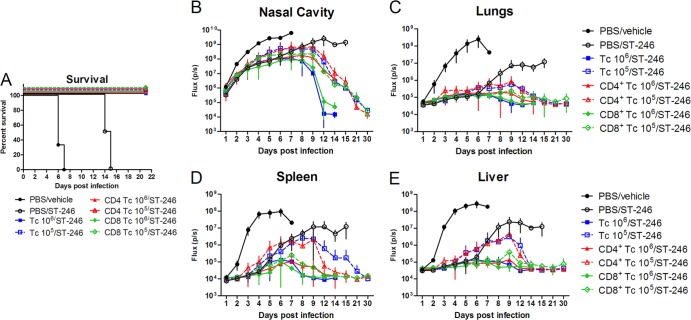
Previous studies in immune-deficient mice showed that continuous administration of ST-246 protected 100% of SCID mice from lethal challenge with VACV-WR (13). However, after ST-246 was stopped, the virus replication ensued, and all mice succumbed. This outcome was predictable based on the virostatic mechanism of ST-246 activity (11). Since most of immune-deficient subjects maintain some functional immune cells, we sought to determine whether treatment with ST-246 can protect T cell-deficient BALB/c nude mice that were reconstituted with T cells. To answer this question, nude mice received an adoptive transfer of 107 T cells purified from the spleens of normal BALB/c mice (or received a sham transfer of PBS) 1 day before infection with 104 PFU of IHD-J-Luc, and were treated daily with vehicle alone (for 7 days), or with ST-246 for 3, 5, or 7 days starting 24 h postinfection (Fig. 4). Nude mice that were reconstituted with T cells or were sham reconstituted with PBS and were treated with vehicle (Tc/vehicle and PBS/vehicle, respectively) had nearly identical lethality curves, and all succumbed by day 12 postinfection (Fig. 4A, closed black and gray circles). Nude mice that did not receive T cells and were treated with ST-246 for 7 days (PBS/ST-246) survived for the duration of treatment but subsequently succumbed to infection between days 12 to 16 (Fig. 4A, open circles). In contrast, the groups of T cell-reconstituted mice that were treated with ST-246 for 3, 5, or 7 days, showed survival rates of 25, 75, and 100%, respectively. These data suggested that a 7-day treatment with ST-246 was required to allow generation of strong antiviral T cell immunity that could control virus replication after treatment cessation (Fig. 4A).
Fig 4.
Nude mice reconstituted with 10 million T cells were protected from lethality when they received ST-246 for no less than 1 week. Nude mice received an adoptive transfer of 10 million purified T cells, were infected with IHD-J-Luc VACV at 104 PFU, and were treated daily with vehicle alone for 7 days (gray circles), or with ST-246 at 100 mg/kg for 7 days (red square), 5 days (blue triangle), or 3 days (inverted green triangle). In control, nude mice received transfer of PBS (sham adoptive transfer), were infected, and were treated daily for 7 days with vehicle alone (black circles) or with ST-246 (black open circles). Mice were observed for lethality (A) and were subjected to bioimaging daily to calculate mean fluxes ± the SD in the nasal cavity (B), lungs (C), spleen (D), and liver (E). PBS/vehicle, Tc/vehicle, Tc/ST-246 5D, and Tc/ST-246 3D, 8 mice per group; PBS/ST-246 and Tc/ST-246 7D, 4 mice per group. Mean background levels of fluxes (p/s, photons per second) ± the SD were recorded in nude mice prior to infection in the nasal cavity (23.3 × 103 ± 4.5 × 103), lungs (36.3 × 103 ± 11.6 × 103), spleen (10.9 × 103 ± 2.5 × 103), and liver (30.2 × 103 ± 2.1 × 103) and are shown as broken lines (means only) in panels B, C, D, and E, respectively. The experiment was performed twice with similar results.
All animals were imaged daily for the first 10 days, and surviving animals were imaged for additional days, as indicated in Fig. 4B to E. Vehicle-treated animals demonstrated the same kinetics of virus replication in all organs, irrespective of whether they were reconstituted with T cells or not, confirming that T cells alone did not control viral dissemination (Fig. 4B to E, black and gray circles). The mean fluxes in the organs of mice that received T cells and ST-246 for 3 or 5 days were similar for both groups early postinfection but increased sharply once ST-246 treatment was terminated, on days 5 and 7, respectively (Fig. 4B to E, green inverted triangles and blue triangles). Importantly, in mice that received 7-day treatment with ST-246 with or without T cells, viral loads were controlled until day 8 (the last time point of ST-246 treatment) and then sharply increased in mice that received sham transfer (open circles). In nude mice that received adoptively transferred T cells and that were treated with ST-246 (Tc/ST-246) for 7 days (red squares) bioluminescence was reduced compared to PBS/vehicle or Tc/vehicle groups and returned to levels of bioluminescence in noninfected animals by day 13, suggesting clearance of the virus. Surviving animals in the Tc/ST-246 shorter treatment groups (3 or 5 days) also cleared the virus from all organs between days 13 and 21 (Fig. 4B to E, green and blue triangles and data not shown).
To further analyze the contributions of T cells and of ST-246 on viral loads, mean AUCs were calculated for fluxes in the organs of individual mice for either the first 6 days (time period when all mice survived), the first 8 days (when all mice in groups with ST-246 treatment survived), or the first 13 days (when all mice in the PBS/ST-246 [7-day] and Tc/ST-246 [7-day] treatment groups survived) (Table 4). On day 6 postchallenge, a significant difference in mean AUC in all organs was observed between mice that were treated with ST-246 with or without T cell transfer compared to the control group (PBS/vehicle). On days 8 and 13, we compared the AUCs between groups of mice that were treated with ST-246 with or without T cell transfer (i.e., Tc/ST-246 versus PBS/ST-246). On day 8, mean AUCs were significantly higher in the lungs, spleen, and liver in mice that were reconstituted with T cells and treated with ST-246 for only 3 days compared to AUCs in nonreconstituted mice that received ST-246 treatment for 7 days, suggesting that at early time points after infection, ST-246 plays a dominant role in reducing viral loads. Importantly, at day 13, in mice that received ST-246 for 7 days, mean AUCs were significantly lower in all organs of T cell-reconstituted mice compared to the AUCs in nonreconstituted mice, suggesting that after ST-246 was stopped (day 8), T cells were able to control viral loads and eventually clear virus-infected cells (Table 4 and Fig. 4B to E).
Table 4.
Mean AUCs in the organs of nude mice reconstituted with 10 million T cells and infected with IHD-J-Luc
| Organ and treatment | Mean AUC (log10 p/s × day) ± 2×SEa |
||
|---|---|---|---|
| D6 | D8 | D13 | |
| Nasal cavity | |||
| PBS/vehicle | 43.2 ± 1.6 | ||
| Tc/vehicle | 43.0 ± 2.3 | ||
| PBS/ST-246 | 40.4 ± 2.0* | 57.5 ± 2.8 | 103.0 ± 3.7 |
| Tc/ST-246 (7 days) | 38.6 ± 0.5* | 55.1 ± 1.1 | 86.8 ± 3.8* |
| Tc/ST-246 (5 days) | 39.1 ± 1.9* | 57.0 ± 2.5 | |
| Tc/ST-246 (3 days) | 40.9 ± 0.9* | 45.5 ± 0.9* | |
| Lungs | |||
| PBS/vehicle | 33.7 ± 1.2 | ||
| Tc/vehicle | 34.4 ± 1.0 | ||
| PBS/ST-246 | 28.9 ± 0.6* | 40.7 ± 0.9 | 73.9 ± 2.1 |
| Tc/ST-246 (7 days) | 27.4 ± 0.7* | 38.4 ± 0.8* | 62.5 ± 1.4* |
| Tc/ST-246 (5 days) | 28.2 ± 0.7* | 40.6 ± 0.9 | |
| Tc/ST246 (3 days) | 29.2 ± 0.9* | 43.3 ± 1.1* | |
| Spleen | |||
| PBS/vehicle | 32.7 ± 1.3 | ||
| Tc/vehicle | 33.5 ± 1.7 | ||
| PBS/ST-246 | 24.9 ± 0.7* | 36.2 ± 0.9 | 69.2 ± 0.9 |
| Tc/ST-246 (7 days) | 25.3 ± 0.8* | 35.4 ± 1.0 | 56.5 ± 1.3* |
| Tc/ST-246 (5 days) | 25.9 ± 0.7* | 38.9 ± 0.7* | |
| Tc/ST-246 (3 days) | 27.1 ± 0.5* | 42.2 ± 1.0* | |
| Liver | |||
| PBS/vehicle | 35.5 ± 1.2 | ||
| Tc/vehicle | 35.6 ± 1.4 | ||
| PBS/ST-246 | 28.0 ± 0.9* | 40.1 ± 1.6 | 75.3 ± 2.2 |
| Tc/ST-246 (7 days) | 27.2 ± 0.8* | 38.3 ± 1.3 | 63.1 ± 2.2* |
| Tc/ST-246 (5 days) | 27.7 ± 0.8* | 41.2 ± 1.0 | |
| Tc/ST-246 (3 days) | 28.9 ± 0.4* | 44.6 ± 0.7* | |
The mean AUC values were calculated for fluxes in mice for days 1 to 6 (D6), 1 to 8 (D8), and 1 to 13 (D13) for groups where all mice survived during this time frame. For D6, the significance is indicated between the mean AUCs in T cell/vehicle (Tc/vehicle) or T cell/ST-246 (Tc/ST-246) groups versus the PBS/vehicle group (indicated in boldface); for D8 and D13, significance is indicated between the mean AUCs for PBS/ST-246 (indicated in boldface) versus the Tc/ST-246 groups.
, P ≤ 0.05. p/s, photons per second.
Dorsal images were used to score poxes on mouse tails (Table 5). Poxes were detected starting from day 4 and peaked around days 8 to 9 in both PBS/vehicle and Tc/vehicle groups, indicating that T cells alone could not prevent pox development. In sham-reconstituted mice, ST-246 inhibited poxes during treatment; however, poxes were detected after ST-246 was stopped (day 12). In T-cell reconstituted mice that received ST-246 for 3 or 5 days poxes started to appear after ST-246 was stopped, and their numbers increased in the following days up to the levels seen in vehicle-treated mice. Importantly, in T cell-reconstituted mice that received ST-246 for 7 days, no poxes were detected on day 12, suggesting that T cells controlled both virus replication in internal organs (Fig. 4) and dissemination to the skin (Table 5).
Table 5.
Pox counts in nude mice reconstituted with T cells, infected with IHD-J-Luc VACV, and treated postchallenge with ST-246 for 3, 5, or for 7 days
| Treatment | Avg pox no./animal/day ± SDa at day postinfection: |
||||||
|---|---|---|---|---|---|---|---|
| 4 | 5 | 6 | 7 | 8 | 9 | 12 | |
| PBS/vehicle | 0.1 ± 0.4 | 2.5 ± 2.1 | 3.5 ± 2.8 | 5.4 ± 2.3 | 5.3 ± 3.2 | 7.0 ± 2.8 | NA |
| Tc/vehicle | 0.3 ± 0.5 | 2.4 ± 1.4 | 3.8 ± 2.4 | 5.2 ± 2.0 | 5.3 ± 5.1 | 2.0 ± 0.0* | NA |
| PBS/ST-246 (7 days) | 0 | 0* | 0* | 0.3 ± 0.5* | 0* | 0* | 4.8 ± 1.0 |
| Tc/ST-246 (7 days) | 0 | 0* | 0* | 0.5 ± 0.6* | 0.5 ± 0.6* | 0.5 ± 1.0* | 0 |
| Tc/ST-246 (5 days) | 0 | 0* | 0* | 0.5 ± 0.8** | 1.5 ± 1.4* | 1.5 ± 1.3* | 2.1 ± 1.9 |
| Tc/ST-246 (3 days) | 0 | 0* | 0.5 ± 0.8* | 0.2 ± 1.1* | 4.9 ± 1.6 | 8.0 ± 0.0 | 5.0 ± 1.4 |
Asterisks denote significant differences between PBS/vehicle and all other groups in mean daily pox count.
, P ≤ 0.05. NA, not applicable.
Together, these data demonstrated that neither T cells nor a 7-day treatment with ST-246 alone could rescue nude mice from fatal vaccinia virus infection. However, immune reconstitution of nude mice with T cells (107 per animal) reduced viral dissemination, prevented pox lesion development, and protected from lethality, if viral replication was controlled by ST-246 for no less than 1 week.
Either CD4+or CD8+ T cell reconstitution can provide long-term protection of ST-246-treated nude mice from lethal VACV challenge.
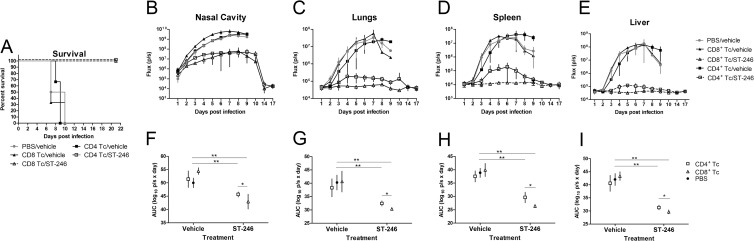
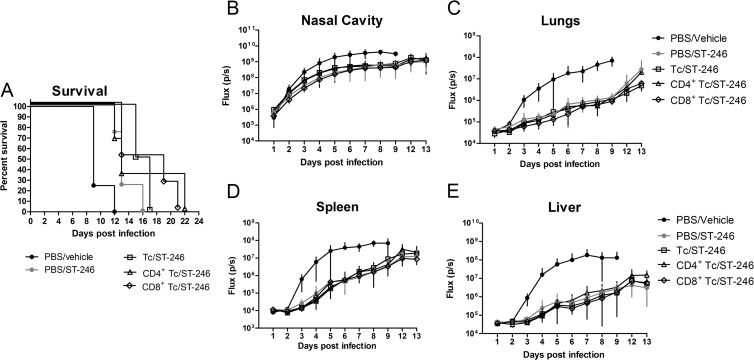
To determine whether CD8+ or CD4+ T cells are required for protection of nude mice from lethal challenge with VACV, we purified CD4+ T cells and CD8+ T cells from the spleens of normal BALB/c mice and injected 10 million cells of either subset into the mouse tail vein 1 day prior to infection with 104 PFU of IHD-J-Luc. Starting at 24 h postinfection, mice received daily treatments with ST-246 at 100 mg/kg or vehicle for 7 days (Fig. 5). As seen in the previous experiments, no vehicle-treated animals (with or without T cell reconstitution) were protected from lethality (Fig. 5A, closed symbols). In contrast, all mice that were reconstituted with either CD4+ T cells or CD8+ T cells before challenge and were treated with ST-246 for 7 days survived (Fig. 5A, open symbols). Imaging of mice showed that fluxes in the nasal cavity, lungs, spleen, and liver of all vehicle-treated groups increased sharply starting from 2 days postinfection (Fig. 5B to E, closed symbols). In contrast, mice that were reconstituted with T cell subsets and were treated with ST-246, exhibited good control over viral replication in the lungs, spleen, and liver, and to a lesser degree in the nasal cavity (Fig. 5B to E, open symbols). Mean AUCs ± 2 × the standard error (2×SE) were calculated based on the AUCs of fluxes measured in individual mice for first 7 days, the last time point where all animals survived (Fig. 5F to I). In all four organs, the mean AUCs were not significantly different between control mice (PBS/vehicle) and mice reconstituted with either CD4+ or CD8+ T cells and treated with vehicle, suggesting that neither subset was sufficient to suppress viral replication in the absence of antiviral treatment (Fig. 5F to I). In contrast, in mice that were reconstituted with CD4+ or with CD8+ T cells and were treated with ST-246 (7 days), AUC values were significantly lower compared to control mice (Fig. 5F to I). In addition, in ST-246 treatment groups, there was a trend toward lower AUCs in mice reconstituted with CD8+ T cells compared to CD4+ T cells that reached significance (Fig. 5F to I).
Fig 5.
Adoptively transferred CD4+ and CD8+ T cells conferred protection from lethality and reduced viral loads in the recipient nude mice after ST-246 treatment. Nude mice received an adoptive transfer with 10 million of CD8+ T cells (closed and open triangles) or CD4+ T cells (closed and open squares) or were sham transferred (gray circles) (A to E). All mice were infected with 104 PFU of IHD-J-Luc and were treated daily with ST-246 at 100 mg/kg (closed triangles and closed squares) or with equal volume of vehicle (gray circles, open triangles, and open squares) for 7 days starting 24 h postchallenge (A to E). Mice were observed for lethality (A) and were subjected to bioimaging (B to E). Total fluxes in the nasal cavity (B), lungs (C), spleen (D), and liver (E) were determined and used to calculate mean total flux ± the SD using a Student t test. (F to I) AUCs were calculated for fluxes from individual mice for first 7 days in the nasal cavities (F), lungs (G), spleens (G), and livers (H) and used to calculate mean AUCs ± 2×SE for mice that received sham transfer with PBS (black circles) or were reconstituted with CD4+ (open squares) or CD8+ T cells (open triangles). Significant differences are shown between CD4+ or CD8+ T cell-reconstituted and ST-246-treated mice and sham-reconstituted vehicle-treated mice and for ST-246-treated nude mice reconstituted with CD4+ versus CD8+ T cells. *, P ≤ 0.05; **, P ≤ 0.001. PBS/vehicle, CD8+ Tc/ST-246, 4 mice per group; CD4+ Tc/vehicle and CD8+ Tc/vehicle, 3 mice per group; CD4+ Tc/ST-246, 5 mice per group. The experiment was performed twice with similar results.
VACV-induced lesions were scored on the tails of nude mice, and the results showed that similar to animals reconstituted with total T cells (Table 5), in the absence of ST-246 treatment, neither CD4+ nor CD8+ T cells were able to prevent pox development (Table 6). However, in the presence of ST-246 treatment, no poxes were detected in infected nude mice that were reconstituted with CD4+ or with CD8+ T cells even after the ST-246 treatment ceased (Table 6).
Table 6.
Pox counts in nude mice reconstituted with CD4+ or with CD8+ T cells, infected with IHD-J-Luc VACV, and subjected to treatment with ST-246 for 7 days
| Treatment | Avg pox no./animal/day ± SDa at day postinfection: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 4 | 5 | 6 | 7 | 8 | 9 | 10 | 14 | 17 | |
| PBS/vehicle | 0 | 2.8 ± 1.0 | 5.0 ± 1.6 | 7.3 ± 1.7 | 8.0 ± 0.0 | 9.0 ± 1.4 | NA | NA | NA |
| CD8+ Tc/vehicle | 1.0 ± 1.0 | 3.3 ± 1.5 | 5.3 ± 0.6 | 6.7 ± 1.2 | 7.0 ± 0.0 | 10.0 ± 0.0 | NA | NA | NA |
| CD8+ Tc/ST-246 | 0 | 0** | 0** | 0** | 0** | 0** | 0 | 0 | 0 |
| CD4+ Tc/vehicle | 1.0 ± 0.0 | 1.7 ± 1.2* | 4.0 ± 1.0 | 4.7 ± 1.5 | 7.7 ± 2.1 | NA | NA | NA | NA |
| CD4+ Tc/ST-246 | 0 | 0** | 0** | 0** | 0** | 0** | 0 | 0 | 0 |
Asterisks denote significant differences between PBS/vehicle and all other groups in mean daily pox count.
, P ≤ 0.05;
, P ≤ 0.001. NA, not applicable.
Titration of transferred T cells in nude mice prior to IHD-J-Luc VACV challenge and ST-246 treatment.
In order to better mimic patients with different levels of T cell deficiency, it was important to identify the lowest number of transferred T cells sufficient to protect nude mice from lethal challenge with VACV in the presence of ST-246. To that end, nude mice received an adoptive transfer with 1.0, 0.1, and 0.01 million of total T cells or CD4+ or CD8+ T cells purified from the spleens of normal BALB/c mice, or received a sham transfer with PBS (Fig. 6, 7, and 8). One day postreconstitution, mice were infected with 104 PFU of IHD-J-Luc and were treated with vehicle or with ST-246 daily for 7 days starting 24 h postinfection. As in previous experiments, control mice (PBS/vehicle) succumbed between days 6 and 12, whereas mice that were treated with ST-246 (PBS/ST-246) succumbed between days 12 and 16 (Fig. 6A). After an adoptive transfer of T cells or of CD4+ or CD8+ T cells at 106 or 105 cells per mouse and treatment with ST-246 for 1 week, nude mice survived (Fig. 6, closed and open colored symbols), did not loose weight (data not shown), and cleared the viral infection in all organs by day 14 or by day 30, respectively.
Fig 6.
One million and 0.1 million of T cells transferred to nude mice were sufficient to protect from lethality in conjunction with 7-day treatment with ST-246. One day prior to infection with 104 PFU of IHD-J-Luc, nude mice received an adoptive transfer with 1.0 or 0.1 million of T cells (blue closed and open squares) or of CD4+ (red closed and open triangles) or CD8+ T cells (green closed and open diamonds) from normal BALB/c mice and starting 1 day postinfection, were all treated with ST-246 for 7 days. Control mice were sham transferred with PBS 1 day before infection and after infection received vehicle or ST-246 (black closed and open circles) for 7 days. Mice were observed for lethality (A) and were subjected to bioimaging until day 14 or 15 for all surviving mice and additionally on days 21 and 30 for the group of mice that were reconstituted with 0.1 million of T cells or T cell subsets to confirm complete clearance of IHD-J-Luc from all organs. Recorded fluxes were used to calculate mean fluxes ± the SD in the nasal cavity (B), lungs (C), spleen (D), and liver (E). PBS/vehicle, 3 mice per group; PBS/ST-246, Tc 106/ST-246, and Tc 105/ST-246, 4 mice per group. The experiment was performed twice with similar results.
Fig 7.
Ten thousand T cells or CD4+ or CD8+ T cells transferred into nude mice did not protect from lethality and from viral dissemination. Nude mice received a sham transfer with PBS (black and gray circles) or with 104 T cells (open squares), CD4+ T cells (open triangles), or CD8+ T cells (open diamonds). All mice were infected with 104 PFU of IHD-J-Luc 1 day after reconstitution and treated with vehicle (black circles) or with ST-246 (gray circles and open symbols) daily for 7 days starting 24 h postinfection. Mice were observed for lethality (A) and were subjected to bioimaging daily to calculate mean fluxes ± the SD in the nasal cavity (B), lungs (C), spleen (D), and liver (E). Four mice per group were used in the experiment.
Fig 8.
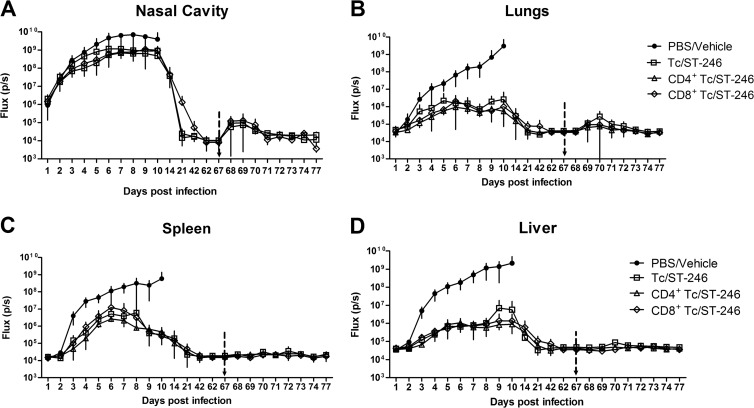
Nude mice reconstituted with 0.1 million of T cells or of CD4+ or CD8+ T cells were protected from rechallenge with IHD-J-Luc without the need for additional ST-246 treatment. Nude mice were sham transferred with PBS (●) or received adoptive transfer with 0.1 million of T cells (□) or of CD4+ (△) or CD8+ T cells (♢) and were infected with 104 PFU IHD-J-Luc 1 day after reconstitution. Sham-transferred mice received vehicle (PBS/vehicle) and T cell reconstituted mice received ST-246 for 7 days (Tc/ST-246, CD4+ Tc/ST-246, and CD8+ Tc/ST-246). On day 67 postinfection, all survived mice were rechallenged with 104 PFU of IHD-J-Luc. Mice were imaged after primary infection up to day 62 at time points indicated on the x axis and then daily for 10 days after rechallenge. Recorded fluxes were used to calculate mean fluxes ± the SD in the nasal cavity (A), lungs (B), spleen (C), and liver (D). Vertical arrow indicates time of rechallenge on day 67. Four mice per group were used in all groups. The experiment was performed twice with similar results.
To determine whether the differences in viral loads in mice that received 1.0 or 0.1 million T cells or T cell subsets were significant, AUCs for fluxes in individual mice were calculated for days 1 to 6 (all groups) and for days 1 to 12 (only for mice in ST-246-treated groups) and used to calculate mean AUCs ± 2×SE (Table 7). On day 6, mean AUCs were significantly lower in all four organs of mice that received ST-246 compared to vehicle-treated mice. There were no significant differences between mice that received ST-246 with or without T cells on day 6 (data not shown). On the other hand, in the ST-246 treatment group, significant differences in the mean AUCs were observed between mice reconstituted with 106 total T cells or with CD4+ or CD8+ T cells versus nonreconstituted mice on day 12 when ST-246 treatment ended but all mice in ST-246 groups were alive. In the case of transfer of 105 cells, total T cells or CD8+ T cells controlled viral loads after ST-246 was terminated as efficiently as 106 transferred cells in the lungs, spleen, and liver. However, reconstitution with 105 of CD4+ T cells did not significantly reduce AUCs, suggesting that 105 of CD4+ T cells did not control viral loads to the same degree as 105 total T cells or CD8+ T cells (Table 7, D12 columns). Together, comparisons of mean AUCs of fluxes between groups of reconstituted mice suggested that 1.0 million T cells or T cell subsets were more efficient than 0.1 million in controlling viral loads during and after treatment termination (Fig. 6 and Table 7).
Table 7.
Mean AUC values in nude mice reconstituted with low numbers of T cells, infected with IHD-J-Luc VACV, and treated with ST-246 for 7 days
| Adoptive transfer/treatment | No. of adoptively transferred cells | Mean AUC (log10 p/s × day) ± 2×SEa |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Nasal cavity |
Lungs |
Spleen |
Liver |
||||||
| D6 | D12 | D6 | D12 | D6 | D12 | D6 | D12 | ||
| PBS/vehicle | 45.4 ± 0.3 | 36.9 ± 1.8 | 35.4 ± 0.6 | 37.2 ± 0.7 | |||||
| PBS/ST-246 | 39.0 ± 0.5** | 92.0 ± 2.9 | 26.6 ± 0.5** | 64.9 ± 1.7 | 23.9 ± 1.8** | 63.3 ± 3.8 | 26.1 ± 0.8** | 67.0 ± 2.3 | |
| Tc/ST-246 | 106 | 39.7 ± 1.3** | 78.7 ± 3.6** | 27.2 ± 0.7** | 55.9 ± 1.2** | 23.9 ± 1.0** | 49.4 ± 2.0** | 26.1 ± 0.5** | 54.7 ± 1.7** |
| Tc/ST-246 | 105 | 39.5 ± 2.9* | 87.8 ± 5.8 | 27.0 ± 0.6** | 59.8 ± 2.0* | 24.6 ± 1.1** | 57.4 ± 4.2 | 26.5 ± 1.4** | 60.5 ± 4.6* |
| CD4+ Tc/ST-246 | 106 | 40.1 ± 1.2** | 85.9 ± 3.2* | 27.4 ± 0.9** | 58.0 ± 1.7** | 23.2 ± 1.4** | 49.9 ± 2.9** | 26.0 ± 0.8** | 55.0 ± 1.5** |
| CD4+ Tc/ST-246 | 105 | 39.7 ± 2.5* | 89.1 ± 7.0 | 27.9 ± 1.6** | 60.3 ± 5.2 | 25.3 ± 2.2** | 56.8 ± 7.8 | 27.1 ± 1.4** | 60.6 ± 6.4 |
| CD8+ Tc/ST-246 | 106 | 38.4 ± 1.8** | 78.2 ± 5.4* | 27.3 ± 0.5** | 56.4 ± 0.5** | 23.3 ± 0.7** | 48.7 ± 1.0** | 26.0 ± 0.3** | 54.6 ± 1.2** |
| CD8+ Tc/ST-246 | 105 | 40.4 ± 1.2** | 87.3 ± 3.8 | 26.9 ± 0.6** | 57.5 ± 2.1* | 23.9 ± 0.9** | 52.2 ± 1.4* | 26.0 ± 0.3** | 57.0 ± 0.1** |
The mean AUC values were calculated for fluxes in mice for days 1 to 6 (D6) and days 1 to 12 (D12) for groups where all of the mice survived during this time frame. For D6, the significance is indicated between mean AUC in PBS/vehicle (boldface values) versus the PBS/ST-246 or Tc/ST-246 groups; for D12, significance is indicated between the PBS/ST-246 (boldface values) and Tc/ST-246 groups. PBS/vehicle, 3 mice per group; CD4+ Tc 106/ST-246, 5 mice/group, all other groups, 4 mice per group.
, P ≤ 0.05;
, P ≤ 0.001. p/s, photons per second.
The lowest number of T cells or T cell subsets used for reconstitution, 104 cells, failed to rescue mice from lethality, and the viral loads in all organs continued to increase during and after the 7-day ST-246 treatment (Fig. 7). Therefore, we have identified the 105 T cell as the lowest cell number required for full protection from lethality, as well as virus control and prevention of poxes.
Reconstituted nude mice survived VACV infection and developed long-term VACV-specific memory. To determine whether reconstitution of nude mice with T cells (or CD4+ or CD8+ T cell subsets) was sufficient not only to clear the primary virus challenge but also to generate long-term VACV-specific immunity, we repeated the experiment described in Fig. 6 and reconstituted nude mice with 105 T cells or T cell subsets prior to infection and treatment with ST-246. The viral loads from the primary infection were completely cleared in all organs in all mice by week 3 postinfection (Fig. 8). On day 67 postinfection, all mice were rechallenged with 104 PFU of IHD-J-Luc and subjected to bioimaging for the following 10 days. No weight loss was noted in mice after rechallenge with IHD-J-Luc (data not shown). In the nasal cavities of all mice, a transient increase in fluxes were measured within 1 to 2 days after rechallenge that returned to background levels within 4 days (Fig. 8A). There was minimal dissemination to the lungs (Fig. 8B) and no viral dissemination to the spleen and liver (Fig. 8C and D). These data confirmed that primary infection in T cell-reconstituted and ST-246-treated mice was sufficient to generate virus-specific memory T cells that protected mice from lethality and viral dissemination after secondary infection with no need for additional ST-246 treatment.
DISCUSSION
ST-246 is a low-molecular-weight compound that inhibits the egress of orthopoxviruses from infected cells in vitro and confers protection from orthopoxvirus infections in vivo in animal models. In the present study, we subjected normal and immune-deficient mice infected with a lethal dose of IHD-J-Luc VACV to whole-body bioimaging and assessed the effects of ST-246 on viral loads by comparing mean AUCs between treated and control groups. The main results of the study were as follows: (i) 2 days of treatment with a full dose (100 mg/kg) and 5 days of treatment with a suboptimal dose (30 mg/kg) of ST-246 were required to protect normal mice from lethality and to significantly reduce viral loads (mean AUCs) in the spleen, liver, lungs, and nasal cavity; (ii) treatment with ST-246 could be delayed for up to 3 but not for 4 days without compromising survival, albeit a 3-day delay did not prevent pox development posttreatment; (iii) immune-deficient (nude) mice that were transiently treated with ST-246 or received an adoptive transfer with 10 million T cells did not survive challenge. However, a combination of transferred T cells or of purified CD4+ or CD8+ T subsets 1 day before challenge, followed by a 7-day treatment with ST-246 (starting at 24 h postinfection) protected nude mice from lethality, reduced viral loads in internal organs, and prevented the development of pox lesions; (iv) 0.1 million was found to be the lowest number of T cells required for reconstitution resulting in protection from lethality when combined with ST-246 treatment postchallenge; and (v) surviving nude mice were protected from a subsequent IHD-J-Lu challenge with no need for drug intervention.
Previous studies have shown that oral administration of ST-246 for 2 weeks protected normal mice from lethal challenge with the Western Reserve (WR) or IHD-J strains of VACV (11, 12). Here, we confirmed and expanded these findings by monitoring the replication of IHD-J-Luc VACV containing higher fractions of the EEV form of VACV compared to the WR strain. ST-246 is administered orally. The finding that full dose of ST-246 was also effective in inhibiting viral loads in the lungs and nasal cavity suggested an efficient absorption of the drug from the gastrointestinal tract and thus a favorable bioavailability, including in the mucosal sites of the upper and lower respiratory tracts.
Delayed ST-246 treatment, starting on day 2 or 3 postchallenge was still effective in rescuing mice from VACV-induced lethality, in agreement with previous studies performed in a cowpox virus challenge model (12). However, whole-body bioimaging allowed us to investigate more closely the impact of delayed treatment initiation. It was found that even though both groups of mice survived (2-day or 3-day treatment delay), a significant difference was observed in viral loads in all organs. Viral loads and pox development were much less reduced in animals that started treatment on day 3 compared to day 1 or 2. Moreover, a 4-day delay resulted in no protection from lethality and morbidity.
We also evaluated the effect of low (suboptimal) dose of ST-246 (30 mg/kg). Interestingly, a low dose of ST-246 administered for no less than 5 days protected 100% of mice from lethality. However, when the mean AUCs for 5 days were compared between the full dose and the suboptimal dose groups, a significant difference was observed, suggesting that the suboptimal dose was less efficient at inhibiting viral loads during the first week after challenge. In terms of the effects of ST-246 on dissemination of the virus to the skin, only the full (100 mg/kg) dose but not the suboptimal dose (30 mg/kg) of ST-246 completely prevented pox lesions both during and after the discontinuation of ST-246 treatment in mice that were treated for 5 days. Shorter treatments did not prevent poxes. It is likely that high viral loads in the lungs and skin could contribute to individual-to-individual transmission in recipients of suboptimal dose of ST-246.
Altogether, these data suggested that to not only assure animal survival but also to significantly reduce virus replication in key organs and to prevent pox development, a full dose of ST-246 should be administered for no less than 5 days starting no later than day 2 postchallenge. Suboptimal dose or further delay for 1 day may be protective from lethal challenge but is less effective in reducing viral loads and in controlling dissemination of the virus to the skin. It is expected that under a less optimal time course and dose schedule, individuals will continue to be infectious even during treatment and may succumb later due to virus reemergence.
Individuals with known contraindications for active vaccination against smallpox including immunodeficiency are estimated to be close to 25% of the U.S. population (25). The therapeutic window of ST-246 provides a feasible approach to protect such individuals from vaccine related adverse reactions, including generalized vaccinia.
Previous studies have shown that ST-246 significantly extended the survival of nude and SCID mice after lethal challenge with vaccinia virus strain WR and that mice eventually succumbed after therapy was stopped (13). In the present study, we used nude mice that were reconstituted with T cells purified from naive BALB/c mice in order to better mimic different patient groups with various levels of T cell immune deficiencies. Using bioimaging, we were able to demonstrate virus rebound in all organs of nonreconstituted nude mice within 24 to 48 h after the last day of treatment with ST-246. At the same time, in T cell-reconstituted mice, no reduction in viral loads was observed compared to nonreconstituted mice in the absence of ST-246, suggesting that rapid expansion of the initial viral load in the absence of antiviral drug did not allow sufficient time to elicit a protective immune response in the pool of 10 million transferred naive T cells. In contrast, when ST-246 was administered daily for 7 days, the same number of adoptively transferred T cells protected 100% of nude mice from lethality and cleared viral loads in all organs after ST-246 was stopped. Shorter treatments with ST-246 for 3 and 5 days protected only a proportion of reconstituted nude mice. These data clearly showed that ST-246-mediated control of viral expansion early after infection provided the required time frame for maturation and expansion of virus-specific T cells. It also indicated that the virus was not eliminated by the drug, but the low viral loads were sufficient for antigen-driven T cell maturation into effector cells.
Both humoral and cellular immune responses and CD4+ and CD8+ T cells have been shown to play an important role in protection against poxviruses. Cellular immunity contributes to early protection of naive mice from VACV infection, and antibodies play a major role in protection from monkeypox infection in NHP and against lethal challenge in mice (26, 27). In our experiments, purified CD4+ and CD8+ T cells (ranging between 0.1 and 10 million) in combination with 7-day ST-246 treatment were similarly effective in rescuing nude mice from lethality. Our data on the role of CD8+ T cells in protection from respiratory VACV are in agreement with recent studies by Goulding et al. in RAG−/− mice where the adoptive transfer of 5 million naive CD8+ T cells conferred protection from WR VACV respiratory infection (28). In addition, we showed that nude mice were also protected by adoptively transferred CD4+ T cells in combination with ST-246 treatment, suggesting that transferred naive CD4+ T cells may help endogenous naive B cells that are preserved in nude but not in RAG−/− mice to elicit protective antibodies and compensated for the lack of CD8+ T cells. Our data are in agreement with an earlier report where protection from infection in normal mice was abrogated by depletion of CD4+ T cells (29). Evaluation of VACV-specific antibodies by use of a β-galactosidase reporter gene assay (30) showed that neutralizing antibodies were present on days 33 and 66 postinfection in 30 to 50% of mice reconstituted with T cells, with neutralizing-antibody titers (50% inhibitory dose [ID50 values]) of 1:22 to 1:139 and in 30 to 100% of nude mice reconstituted with CD4+ T cells (ID50, neutralizing antibody titers of 1:24 to 1:71). No VACV-neutralizing antibodies were detected in the sera of mice reconstituted with CD8+ T cells (data not shown). Thus, our studies underscore the existence of multiple pathways of protective immune responses against VACV and confirm previous conclusions on the mechanism of ST-246 that was shown to be protective as long as a single T cell component is intact, whether it is CD8+ or CD4+ (28).
The titration of T cells and of T cell subsets showed that 105 T cells was the lowest number of adoptively transferred T cells that protected nude mice in combination with ST-246 treatment. A total of 2 × 105 to 3 × 105 T cells were detected in the spleens of nude mice reconstituted with 104 or 105 T cells at 1 week postinfection, respectively (data not shown), thus confirming that the lack of protection from lethality was not due to a failed transfer of T cells. It is possible that a longer ST-246 treatment of nude mice reconstituted with 104 T cells (or T cell subsets) could rescue them. However, bioimaging of infected nude mice reconstituted with 104 T cells (Fig. 7) showed that the virus replication curves were nearly identical between reconstituted and nonreconstituted mice treated with ST-246, both during and after the termination of treatment. These findings were significantly different from the kinetics of virus replication in animals reconstituted with a 10-fold-higher number of T cells (105) (Fig. 6). The frequency of VACV-specific CD8+ T cell precursors in naive B6.SJL mouse was estimated to be ∼1 in 1,500 (31). In our experiments, we used BALB/c mice and, therefore, it cannot be ruled out that there are strain-related differences in the frequency of VACV-specific naive CD8+ T cells. However, if the numbers of CD8+ precursors are similar between these two strains of mice, then it could be estimated that there are about 22 and 66 naive CD8+ T cell precursors specific to VACV in the pools of 105 total T cells or CD8+ T cells, respectively. Thus, our data suggest that the failure of 104 T cells or CD8+ T cells to protect nude mice might be due to below-threshold levels of VACV-specific CD8+ precursors in the pool of 10,000 cells compared to the 100,000 cells that conferred protection.
The frequency of CD4+ T cells specific to VACV in naive mice is not well defined. Our data showed that when 105 CD4+ T cells were used for adoptive transfer, nude mice survived infection; however, the recorded fluxes in these mice were higher, especially in the spleen and liver, compared to mice reconstituted with 105 CD8+ T cells (Fig. 6). These data suggest that antiviral responses mounted by CD4+ T cells were less efficient than by CD8+ T cells and/or that the threshold frequency of VACV-specific precursors in naive BALB/c mice is lower for CD4+ than for CD8+ T cells.
A second challenge or reconstituted nude mice without ST-246 treatment resulted in complete protection. These findings suggested the presence of long-term VACV-specific T cells that were generated during primary infection and in the presence of ST-246 treatment. Therefore, it seems that an effective recall response against VACV can provide complete protection with no need for further antiviral therapies.
In summary, our study of the VACV dissemination in immune-deficient mice using bioimaging confirmed that transient treatment with ST-246 does not eliminate virus in these animals and that virus rapidly rebounds in all organs once animals are taken off the drug. However, most immune-deficient humans retain some proportion of functional T cells. Under these circumstances, ST-246 can provide a strong shield for initially very few virus-specific naive CD8+ and CD4+ T cell precursors that expand and eventually reach the required numbers of mature effector cells. Importantly, after reconstituted animals were taken off the drug, no virus rebound was detected, suggesting that immune responses generated under transient ST-246 therapy were capable of clearing VACV from internal organs and prevented its dissemination to the skin.
ACKNOWLEDGMENTS
We are grateful to Clement Meseda and Garcia Alonzo for careful reading of the manuscript.
This project has been funded in part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under IAA 224-06-1322, and by the FDA's Medical Countermeasure Initiative (MCMi) fund.
Footnotes
Published ahead of print 6 March 2013
REFERENCES
- 1. Henderson DA, Inglesby TV, Bartlett JG, Ascher MS, Eitzen E, Jahrling PB, Hauer J, Layton M, McDade J, Osterholm MT, O'Toole T, Parker G, Perl T, Russell PK, Tonat K. 1999. Smallpox as a biological weapon: medical and public health management. JAMA 281:2127–2137 [DOI] [PubMed] [Google Scholar]
- 2. Rimoin AW, Mulembakani PM, Johnston SC, Lloyd Smith JO, Kisalu NK, Kinkela TL, Blumberg S, Thomassen HA, Pike BL, Fair JN, Wolfe ND, Shongo RL, Graham BS, Formenty P, Okitolonda E, Hensley LE, Meyer H, Wright LL, Muyembe JJ. 2010. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc. Natl. Acad. Sci. U. S. A. 107:16262–16267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Whitley RJ. 2003. Smallpox: a potential agent of bioterrorism. Antivir. Res. 57:7–12 [DOI] [PubMed] [Google Scholar]
- 4. Amanna IJ, Slifka MK, Crotty S. 2006. Immunity and immunological memory following smallpox vaccination. Immunol. Rev. 211:320–337 [DOI] [PubMed] [Google Scholar]
- 5. Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. 2003. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 9:1131–1137 [DOI] [PubMed] [Google Scholar]
- 6. Baker RO, Bray M, Huggins JW. 2003. Potential antiviral therapeutics for smallpox, monkeypox, and other orthopoxvirus infections. Antivir. Res. 57:13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Belongia EA, Naleway AL. 2003. Smallpox vaccine: the good, the bad, and the ugly. Clin. Med. Res. 1:87–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Neyts J, De Clercq E. 2003. Therapy and short-term prophylaxis of poxvirus infections: historical background and perspectives. Antivir. Res. 57:25–33 [DOI] [PubMed] [Google Scholar]
- 9. Lalezari JP, Stagg RJ, Kuppermann BD, Holland GN, Kramer F, Ives DV, Youle M, Robinson MR, Drew WL, Jaffe HS. 1997. Intravenous cidofovir for peripheral cytomegalovirus retinitis in patients with AIDS. A randomized, controlled trial. Ann. Intern. Med. 126:257–263 [DOI] [PubMed] [Google Scholar]
- 10. Lanier R, Trost L, Tippin T, Lampert B, Robertson A, Foster S, Rose M, Painter W, O'Mahony R, Almond M, Painter G. 2010. Development of CMX001 for the treatment of poxvirus infections. Viruses 2:2740–2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang G, Pevear DC, Davies MH, Collett MS, Bailey T, Rippen S, Barone L, Burns C, Rhodes G, Tohan S, Huggins JW, Baker RO, Buller RL, Touchette E, Waller K, Schriewer J, Neyts J, DeClercq E, Jones K, Hruby D, Jordan R. 2005. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus challenge. J. Virol. 79:13139–13149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Quenelle DC, Buller RM, Parker S, Keith KA, Hruby DE, Jordan R, Kern ER. 2007. Efficacy of delayed treatment with ST-246 given orally against systemic orthopoxvirus infections in mice. Antimicrob. Agents Chemother. 51:689–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grosenbach DW, Berhanu A, King DS, Mosier S, Jones KF, Jordan RA, Bolken TC, Hruby DE. 2010. Efficacy of ST-246 versus lethal poxvirus challenge in immunodeficient mice. Proc. Natl. Acad. Sci. U. S. A. 107:838–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huggins J, Goff A, Hensley L, Mucker E, Shamblin J, Wlazlowski C, Johnson W, Chapman J, Larsen T, Twenhafel N, Karem K, Damon IK, Byrd CM, Bolken TC, Jordan R, Hruby D. 2009. Nonhuman primates are protected from smallpox virus or monkeypox virus challenges by the antiviral drug ST-246. Antimicrob. Agents Chemother. 53:2620–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jordan R, Chinsangaram J, Bolken TC, Tyavanagimatt SR, Tien D, Jones KF, Frimm A, Corrado ML, Pickens M, Landis P, Clarke J, Marbury TC, Hruby DE. 2010. Safety and pharmacokinetics of the antiorthopoxvirus compound ST-246 following repeat oral dosing in healthy adult subjects. Antimicrob. Agents Chemother. 54:2560–2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lederman ER, Davidson W, Groff HL, Smith SK, Warkentien T, Li Y, Wilkins KA, Karem KL, Akondy RS, Ahmed R, Frace M, Shieh WJ, Zaki S, Hruby DE, Painter WP, Bergman KL, Cohen JI, Damon IK. 2012. Progressive vaccinia: case description and laboratory-guided therapy with vaccinia immune globulin, ST-246, and CMX001. J. Infect. Dis. 206:1372–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Luker KE, Hutchens M, Schultz T, Pekosz A, Luker GD. 2005. Bioluminescence imaging of vaccinia virus: effects of interferon on viral replication and spread. Virology 341:284–300 [DOI] [PubMed] [Google Scholar]
- 18. Rodriguez JF, Rodriguez D, Rodriguez JR, McGowan EB, Esteban M. 1988. Expression of the firefly luciferase gene in vaccinia virus: a highly sensitive gene marker to follow virus dissemination in tissues of infected animals. Proc. Natl. Acad. Sci. U. S. A. 85:1667–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zaitseva M, Kapnick SM, Meseda CA, Shotwell E, King LR, Manischewitz J, Scott J, Kodihalli S, Merchlinsky M, Nielsen H, Lantto J, Weir JP, Golding H. 2011. Passive immunotherapies protect WRvFire and IHD-J-Luc vaccinia virus-infected mice from lethality by reducing viral loads in the upper respiratory tract and internal organs. J. Virol. 85:9147–9158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zaitseva M, Kapnick SM, Scott J, King LR, Manischewitz J, Sirota L, Kodihalli S, Golding H. 2009. Application of bioluminescence imaging to the prediction of lethality in vaccinia virus-infected mice. J. Virol. 83:10437–10447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zaitseva M, Kapnick S, Golding H. 2012. Measurements of vaccinia virus dissemination using whole body imaging: approaches for predicting of lethality in challenge models and testing of vaccines and antiviral treatments. Methods Mol. Biol. 890:161–176 [DOI] [PubMed] [Google Scholar]
- 22. Golden JW, Zaitseva M, Kapnick S, Fisher RW, Mikolajczyk MG, Ballantyne J, Golding H, Hooper JW. 2011. Polyclonal antibody cocktails generated using DNA vaccine technology protect in murine models of orthopoxvirus disease. Virol. J. 8:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. R Development Core Team 2011. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org/ [Google Scholar]
- 24. Karupiah G, Coupar B, Ramshaw I, Boyle D, Blanden R, Andrew M. 1990. Vaccinia virus-mediated damage of murine ovaries and protection by virus-expressed interleukin-2. Immunol. Cell Biol. 68(Pt 5):325–333 [DOI] [PubMed] [Google Scholar]
- 25. Kemper AR, Davis MM, Freed GL. 2002. Expected adverse events in a mass smallpox vaccination campaign. Eff. Clin. Pract 5:84–90 [PubMed] [Google Scholar]
- 26. Belyakov IM, Earl P, Dzutsev A, Kuznetsov VA, Lemon M, Wyatt LS, Snyder JT, Ahlers JD, Franchini G, Moss B, Berzofsky JA. 2003. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc. Natl. Acad. Sci. U. S. A. 100:9458–9463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Edghill-Smith Y, Golding H, Manischewitz J, King LR, Scott D, Bray M, Nalca A, Hooper JW, Whitehouse CA, Schmitz JE, Reimann KA, Franchini G. 2005. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat. Med. 11:740–747 [DOI] [PubMed] [Google Scholar]
- 28. Goulding J, Bogue R, Tahiliani V, Croft M, Salek-Ardakani S. 2012. CD8 T cells are essential for recovery from a respiratory vaccinia virus infection. J. Immunol. 189:2432–2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu R, Johnson AJ, Liggitt D, Bevan MJ. 2004. Cellular and humoral immunity against vaccinia virus infection of mice. J. Immunol. 172:6265–6271 [DOI] [PubMed] [Google Scholar]
- 30. Manischewitz J, King LR, Bleckwenn NA, Shiloach J, Taffs R, Merchlinsky M, Eller N, Mikolajczyk MG, Clanton DJ, Monath T, Weltzin RA, Scott DE, Golding H. 2003. Development of a novel vaccinia-neutralization assay based on reporter-gene expression. J. Infect. Dis. 188:440–448 [DOI] [PubMed] [Google Scholar]
- 31. Seedhom MO, Jellison ER, Daniels KA, Welsh RM. 2009. High frequencies of virus-specific CD8+ T-cell precursors. J. Virol. 83:12907–12916 [DOI] [PMC free article] [PubMed] [Google Scholar]