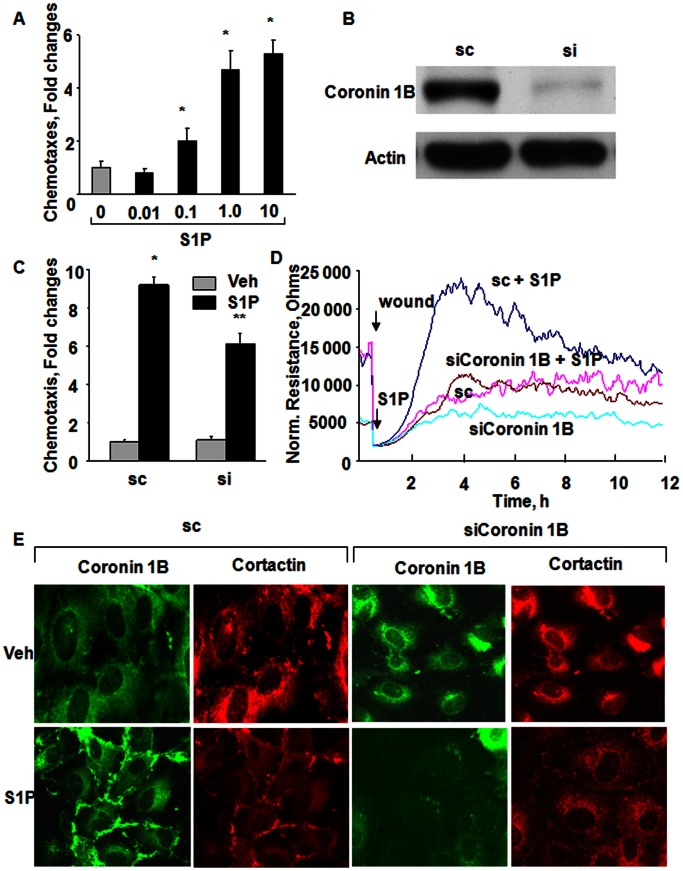
Figure 5. Coronin 1B siRNA attenuates S1P-induced chemotaxis, wound healing and lamellipodia localization of cortactin in HPAECs.
(A), HPAECs grown on transwell inserts were stimulated with different S1P concentration (0.01, 0.1, 1 and 10 µM) for 15 min and chemotaxis was estimated by a Boyden chamber-based trans-well assay as described in Materials and Methods. The values are mean±SEM of three independent experiments. *, p<0.05 compared to cells without S1P. (B) HPAECs were transfected with scrambled (sc) or siRNA for Coronin 1B (50 ng/ml, 72 h), and cell lysates (20 µg of protein) were subjected to 10% SDS-PAGE and probed with Coronin 1B and actin antibodies as indicated. (C) HPAECs grown to 50% confluence in 100-mm dishes were transfected with sc (sc) or Coronin 1B siRNA (50 ng/ml) for 72 h. The cells were trypsinazied and plated on to transwell inserts and S1P-induced chemotaxis was determines as described in (A). The values are mean±SEM of three independent experiments in triplicate. *, p<0.05 compared cells without S1P; **, p<0.001 compared to scrambled siRNA transfected cells plus S1P. (D), HPAECs transfected with scrambled (sc) or Coronin 1B siRNA (50 nM, 72 h) were wounded on the gold electrodes as described under Materials and Methods. Measurement of transendothelial electrical resistance (TER) using an electrical cell substrate impedance-sensing system (ECIS) for 12 h after wounding the cells on the gold electrode and exposure to 1.0 µM S1P was carried out. Shown is a tracing from three independent experiments in triplicate. (E), HPAECs transfected with scrambled (sc) or Coronin 1B siRNA (50 nM, 72 h) were seeded on slide chambers for 24 h prior to stimulation with 1 µM S1P for 15 min. Cells were fixed and Coronin 1B and Cortactin redistribution to cell periphery was visualized by immunocytochemistry as described in Materials and Methods. Shown is a representative immunofluorescence image taken using an X 60 oil objective as described under Materials and Methods.