Table 1.
Affinity, liphopilicity, and molecular weight of pyraxolopyrimidines
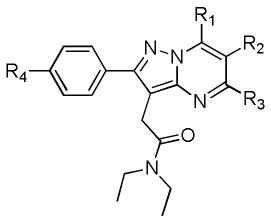
| |||||||
|---|---|---|---|---|---|---|---|
| Compd | R1 | R2 | R3 | R4 | MW | LogP7.5 | Ki (nM)δ |
| 5aα | -Me | -H | -Me | -OMe | 366.21 | 2.40 | 12.23 |
| 5b | -Et | -H | -Et | -OMe | 394.51 | 2.84 | 0.18 |
| 5cβ | -iPr | -H | -iPr | -OMe | 422.56 | 2.98 | >500 |
| 5d | -Me | -Me | -Me | -OMe | 380.48 | 2.45 | 93.75 |
| 5e | -Me | -Et | -Me | -OMe | 394.51 | 2.78 | 157.14 |
| 5f | -Me | -CH2C(O)CH3 | -Me | -OMe | 408.49 | 2.57 | 200.89 |
| 5g | -Me | -Cl | -Me | -OMe | 400.9 | 2.99 | 55.36 |
| 6aγ | -Me | -H | -Me | -OCH2CH2F | 398.47 | 2.12 | 9.73 |
| 6b | -Et | -H | -Et | -OCH2CH2F | 426.53 | 2.50 | 0.27ε |
| 6c | -iPr | -H | -iPr | -OCH2CH2F | 454.58 | 2.73 | >500 |
| 6d | -Me | -Me | -Me | -OCH2CH2F | 412.5 | 2.47 | 83.04 |
| 6e | -Me | -Et | -Me | -OCH2CH2F | 426.53 | 2.53 | 276.79 |
| 6f | -Me | -CH2C(O)CH3 | -Me | -OCH2CH2F | 440.51 | 2.07 | 251.79 |
| 6g | -Me | -Cl | -Me | -OCH2CH2F | 432.92 | 2.55 | 67.86 |
