Figure 2.
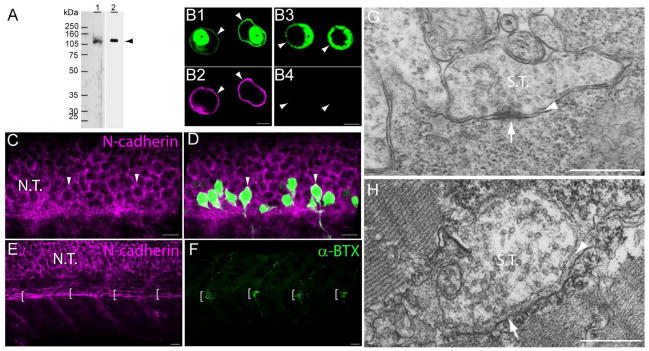
N-cadherin expression in primary motor neurons and muscle pioneer cells. A) Western blot analysis of anti-N-cadherin antibodies (see Table 1). Zebrafish (72hpf) homogenates (40 μg of total protein/lane) were electrophoresed in a 10% SDS-polyacrylamide gel, electro-transferred to a PVDF membrane, and immunoblotted with a rabbit polyclonal anti-zebrafish N-cadherin (lane 1) and MNCD2 monoclonal anti-mouse N-cadherin (lane 2) antibodies. A single band of ~120 kDa was detected in lane 1 and lane 2 by the rabbit polyclonal and MNCD2 antibodies respectively (arrowhead). B) CHO cells were transfected with a plasmid expressing Gal4 under a CMV promoter and a plasmid carrying zebrafish N-cadherin and pren-EGFP under a 14X-UAS element (B1, B2), or a plasmid expressing pren-EGFP under a 14X-UAS (B3, B4). Cells were fixed and immunostained with anti-N-cadherin MNCD2 antibodies and anti-rat IgG Cy3-conjugated secondary antibodies. B1 and B2) confocal images of the same cells showing expression of EGFP (B1) and N-cadherin (B2). B3 and B4) confocal images of the same cells showing expression of EGFP (B3) while no N-cadherin labeling is detected (B4). Arrowheads point to the cell membrane and asterisks indicate the perinuclear region. C and D) Neural tube of 24 hpf Tg(mnx1:GFP) embryos immunostained with anti-N-cadherin MNCD2 antibodies and observed under confocal microscopy. Arrowheads point to primary motor neurons cell bodies labeled with EGFP and expressing N-cadherin on the cell surface. E and F) Wild type zebrafish embryos (24 hpf) double-labeled with anti-N-cadherin MNCD2 antibodies (E) and with α-bungarotoxin (α-BTX) conjugated with Alexa 488 to detect nicotinic acetylcholine receptors (F). Brackets indicate the muscle pioneer cells at the horizontal myoseptum expressing N-cadherin (E), and a distinct cluster of acetylcholine receptors (F). G) Electron micrograph of a neuromuscular junction at the horizontal myoseptum from a 24 hpf wild type zebrafish embryo. The arrowhead points to the synaptic cleft and the arrow to an active zone. H) Electron micrograph of a neuromuscular junction from a 120 hpf wild type zebrafish larvae. The arrowhead points to the synaptic cleft containing a characteristic basal lamina and the arrow points to an active zone determined by the presence synaptic vesicles fused to the presynaptic membrane. Scale bars in B, 5 μm; scale bar in C, D, E and F, 10 μm; scale bar in G and H, 0.5 μm; N.T., Neural Tube; S.T., Synaptic Terminal. Panels C to F, rostral is to the left and dorsal is to the top.
