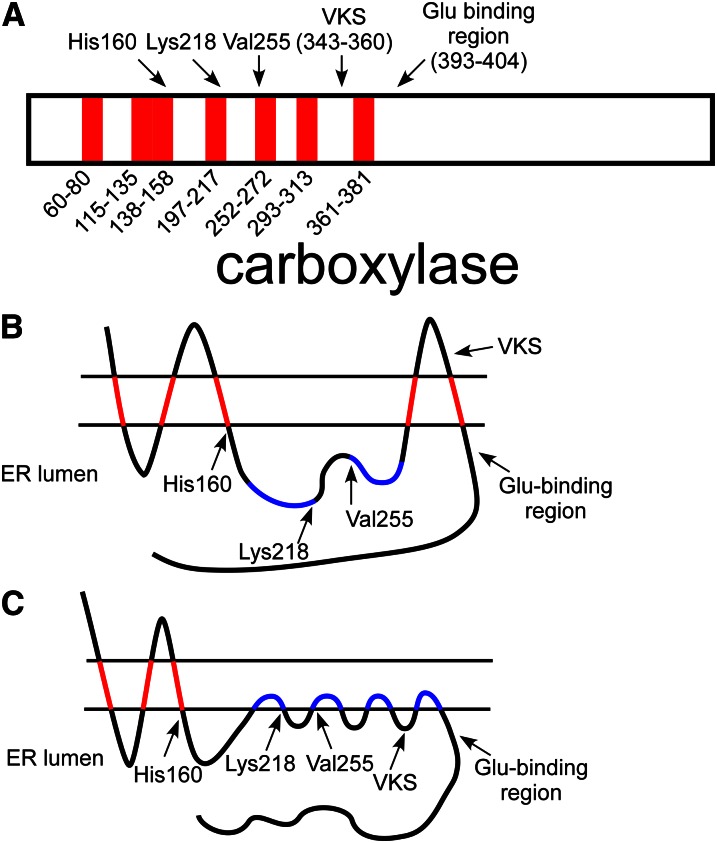
Figure 10.
Topological analysis of the carboxylase. A, Hydropathy analysis of the carboxylase identified 7 candidate transmembrane regions in the carboxylase sequence. Interestingly, several catalytic residues (His160, Lys218, and Val255) are found near the end of these regions. One of the candidate domains is between the vitamin K protein–interacting sequence (VKS) and the glutamate (Glu) binding region. B, An in vitro membrane insertion assay analyzed each domain in isolation and found that 5 of the candidate regions (in red) interacted with membranes, whereas 2 (in blue) did not. Analysis of full-length carboxylase found that the carboxylase N-terminus was cytoplasmic, whereas the C-terminus was lumenal, so that an odd number of transmembrane domains are required. The resulting model, however, places the VKS on the cytoplasmic side of the membrane, whereas the proteins with which this region interacts are lumenal. C, Several of the candidate transmembrane domains (e.g., those in blue) may instead be monotopic, so that they interact with membranes by shallow association with 1 face of the endoplasmic reticulum (ER) membrane. If the most C-terminal candidate region is a monotopic binding domain, then VKS and the Glu-binding region could both be present in the ER lumen for vitamin K–dependent (VKD) protein interaction. The model shown also places 3 residues known to be critical to function in the lumen at the end of membrane-binding regions, suggesting the importance of the membrane surface itself to carboxylase function.