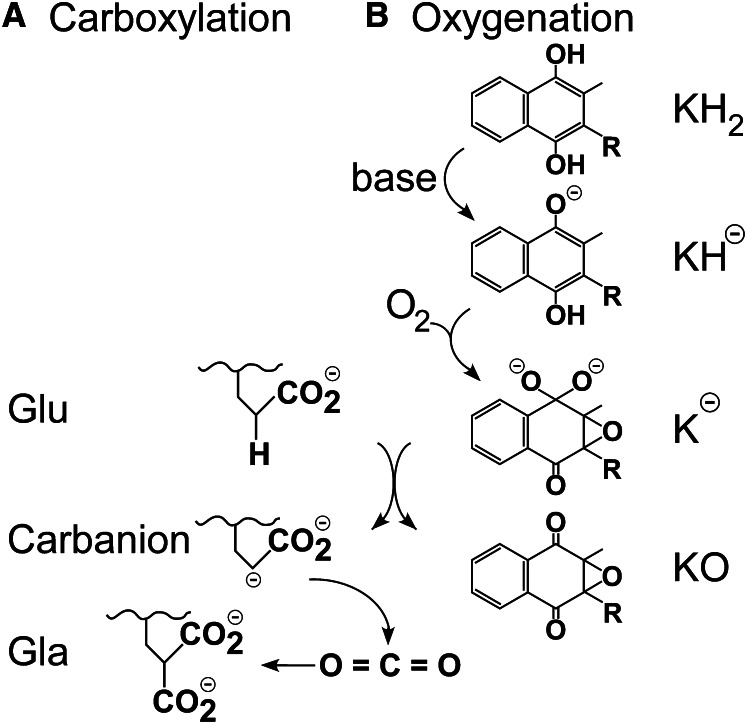
Figure 2.
Glutamate (Glu) deprotonation and carboxylation is accomplished by formation of a strong vitamin K base. A, Glu deprotonation, as opposed to radical formation, was established by experiments with Glu peptide analogs. Carboxylation could either occur in a stepwise process as shown, in which formation of a fully negatively charged carbanion is followed by reaction with CO2 to form γ-carboxyglutamate (Gla), or in a concerted process in which deprotonation and carboxylation are simultaneous and a free, high-energy carbanion is not formed. B, Deprotonation of vitamin K hydroquinone (KH2) by a weak active site base activates the quinone ring for reaction with oxygen, leading to formation of a very strong base species such as K− as shown. This strong base deprotonates Glu, resulting in formation of the vitamin K epoxide product (KO).