Figure 6.
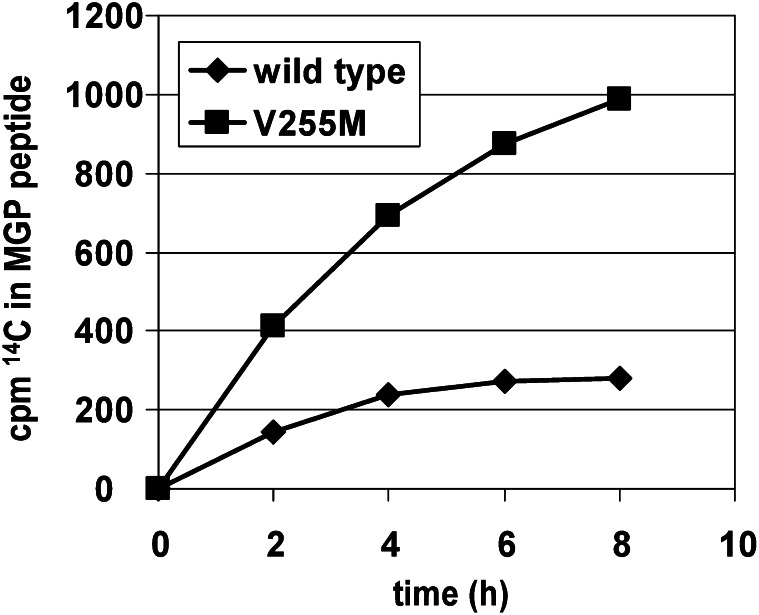
The overall carboxylation rate of a peptide consisting of amino acids 1–64 of matrix γ-carboxyglutamate protein (MGP) is faster with the V255M mutant than with wild type. Almost identical results were observed with substrates containing the propeptide of factor IX linked to either the first 10 Gla domain residues of factor IX or to the entire Gla domain (data not shown). An increased carboxylation rate likely results from faster release of these substrates from V255M, which could lead to impaired processivity. Although overall turnover is faster with V255M, potentially incomplete carboxylation of vitamin K–dependent proteins would be detrimental due to loss of function. MGP, matrix gamma-carboxyglutamate protein.